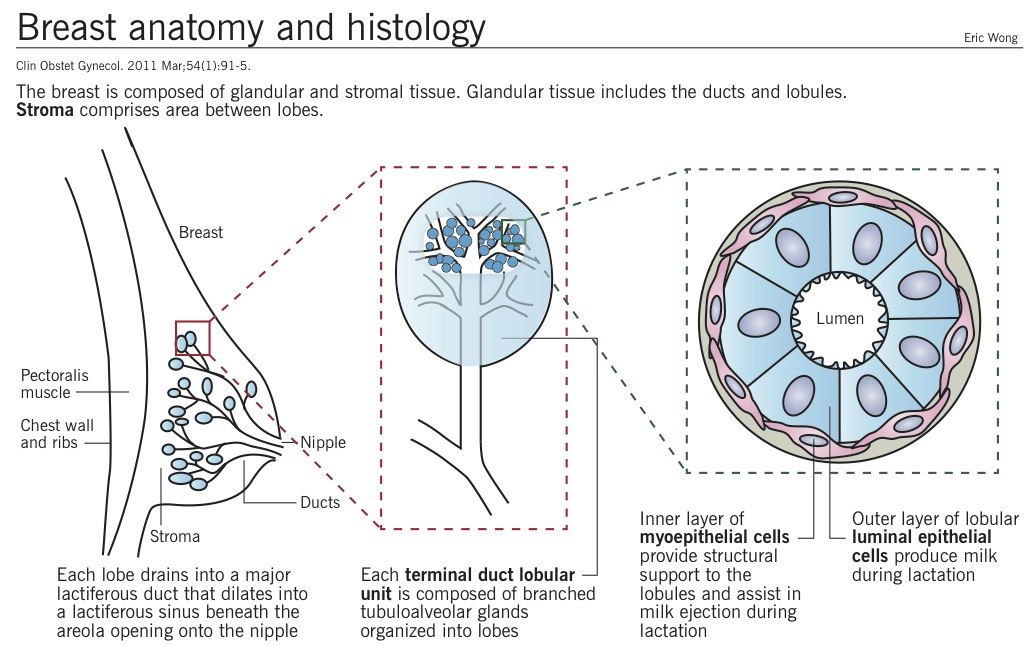
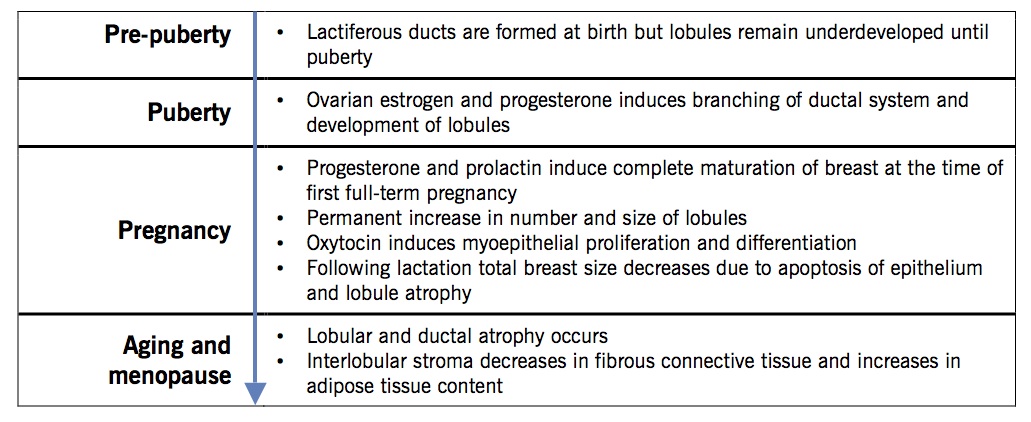
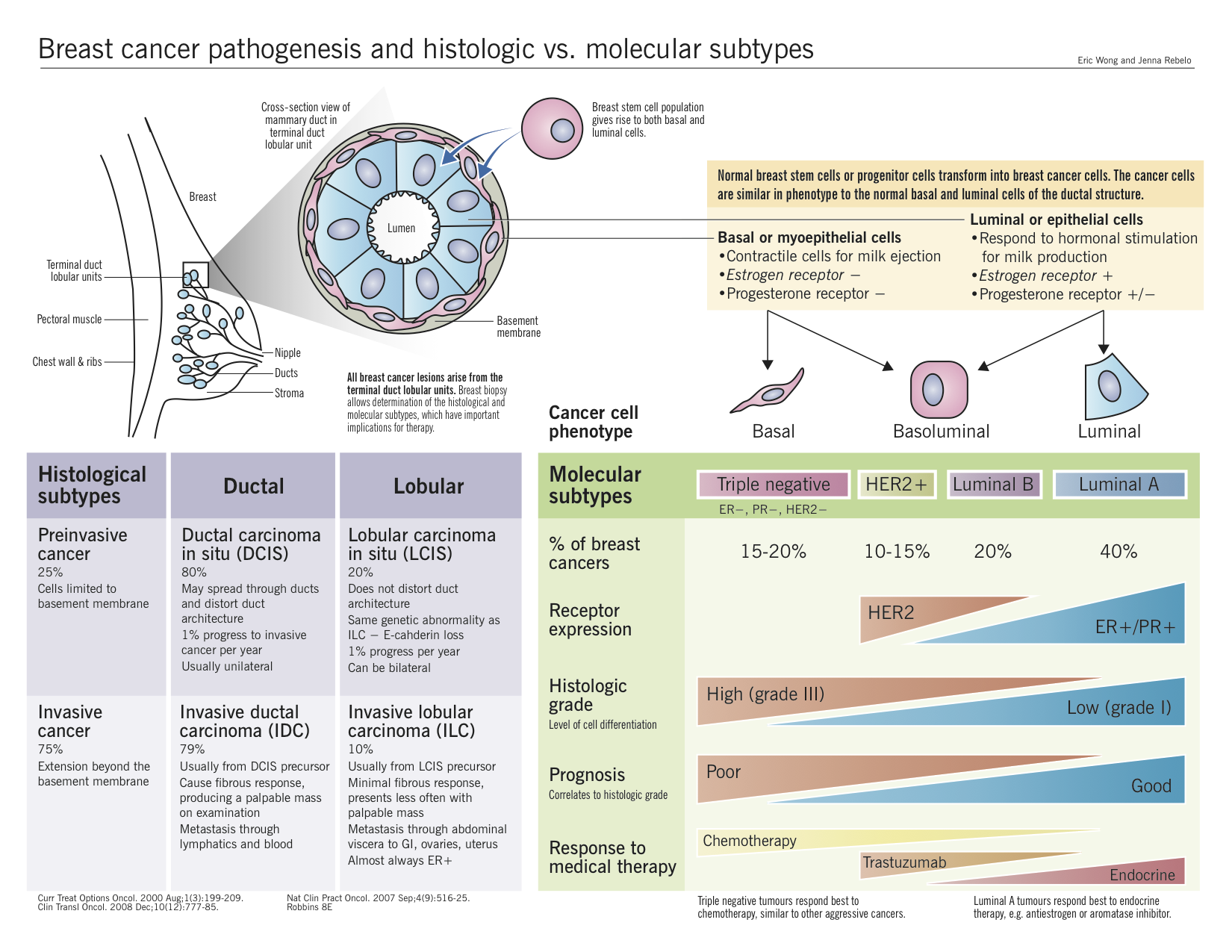
Overview
- Definition: breast cancer refers to several types of neoplasm arising from breast tissue, the most common being adenocarcinoma of the cells lining the terminal duct lobular unit. This chapter only discusses this adenocarcinoma type.
- Breast cancer is the most prevalent cancer in Canadian women, and is the second leading cause of cancer deaths in women. However, the prognosis is good if detected early. The overall 5-year relative survival is 88% in women.
Canadian Cancer Society Statistics 2012 - Hormonal cancer: breast cancer requires a hormonal supply to develop, much like the tissue it arises out of. Risk of breast cancer increases with lifetime estrogen exposure. The majority of breast cancers are hormone sensitive, meaning that they express estrogen receptors and proliferate in response to estrogen stimulation. Endocrine therapies that inhibit estrogen production are effective in treating hormone-sensitive breast cancer.
- Hereditary cancer: approximately 5-10% of breast cancers are hereditary, meaning that there is a known genetic mutation causing increased cancer risk in the patient’s family. Hereditary breast and ovarian cancer (HBOC) syndrome is caused by mutations in two genes, BRCA1 and BRCA2. The genes code for a DNA repair pathway that is important for protecting against mutations. The loss of either gene confers a high risk of breast cancer, as well as other cancers.
Life cycle changes
Risk factors
|
Gender |
Female: Primary risk factor. Lifetime risk in females is 1:8 compared to males at 1:1000. Crit Rev Oncol Hematol. 2010 Feb;73(2):141-55. |
|
Age |
Aging: Risk increases with advancing age. Risk at age 40 is 1:217 and risk at age 80 is 1:10. |
|
Height and weight |
Height: Taller women, both pre- and postmenopausal, have a slightly increased risk; likely correlated with hormonal stimulation. Arch Intern Med. 2006 Nov 27;166(21):2395-402. |
|
Weight: High body-mass index (BMI) is a risk factor for postmenopausal women, likely due to adipose tissue production of estrogen via aromatase. High BMI may lower risk for premenopausal breast cancer due to anovulation (see Polycystic ovarian syndrome chapter) and reduction of circulating estrogen and progesterone. Arch Intern Med. 2007 Oct 22;167(19):2091-102. |
|
|
Medical history |
History of benign proliferative breast disease: Previous breast biopsy showing proliferative changes, particularly with atypical epithelial cells. |
|
History of cancer: Previous history of breast, endometrial or ovarian cancer. |
|
|
Reproductive history |
Early age (<12) at menarche and late age (>55) at menopause: Risk increases linearly with the cumulative number of ovulatory cycles. Proliferation of breast epithelium occurs in the luteal phase of the ovulatory cycle, thereby increasing risk of promotion of initiated cells (see Carcinogenesis chapter). |
|
Late age (>35) of first full-term birth and nulliparity: Pregnancy induces terminal differentiation of luminal cells by exposing the tissue to human chorionic gonadotrophin (hCG). Breast gene expression changes permanently after pregnancy, increasing DNA repair pathways and control over apoptosis. However, pregnancy itself causes a transient risk of breast cancer because of increased estrogen and progesterone exposure, which promotes proliferation in initiated cells. Late age at first full-term birth increases time for exposure to carcinogens. J Mammary Gland Biol Neoplasia. 2011 Sep;16(3):221-33. Nat Rev Cancer. 2006 Apr;6(4):281-91. |
|
|
Exposure history |
Hormone replacement therapy (HRT): Combined estrogen and progesterone therapy has been linked to the development of breast cancer in postmenopausal women; not estrogen alone. Oral contraceptives (OCP) do not increase risk. Estrogen and progesterone in HRT likely promotes preneoplastic lesions rather than initiate them. Since OCP is used in younger women, the number of preneoplastic lesions is much lower than in postmenopausal women, rendering the OCP risk much lower. JAMA. 2003 Jun 25;289(24):3243-53. JAMA. 2010 Oct 20;304(15):1684-92. Nat Rev Clin Oncol. 2011 Aug 2;8(11):669-76. |
|
Ionizing radiation: Breast tissue is sensitive to carcinogenic effects of radiation. Risk is highest in the developing breast and absent after menopause. See Carcinogenesis chapter for mechanism of ionizing radiation. Breast Cancer Res. 2005;7(1):21-32. |
|
|
Smoking: First hand smoking at a young age as well as before a first full term pregnancy. Smoking allows tobacco carcinogens to initiate breast cells prior hormonal stimulation during young adulthood and pregnancy. Cigarette smoke contains at least 20 carcinogens that are known to transform breast cells. Tob Control. 2011 Jan;20(1):e2. BMJ. 2011 Mar 1;342:d1016. |
|
|
Alcohol: Alcohol has been shown to increase the amount of circulating estrogen, possibly by decreasing hepatic metabolism, increasing aromatase activity, or increasing adrenal sex hormone production. JAMA. 2011 Nov 2;306(17):1884-90. |
|
|
Family history
*Autosomal dominant and high penetrance genes
Hematol Oncol Clin North Am. 2010 Oct;24(5):799-814. |
Affected first-degree relatives: Risk increases with number of affected relatives, especially with early-onset breast cancer, bilateral breast cancer or male breast cancer. |
|
Breast cancer predisposition syndromes |
|
|
Hereditary breast and ovarian cancer (HBOC) syndrome: Associated germline mutation intumour suppressor genes BRCA1* and BRCA2* involved in homologous DNA repair. See below for details. |
|
|
Li-Fraumeni syndrome: Characterized by early onset breast cancers, sarcomas, brain tumours, adrenal cortical tumours and acute leukemias. Associated germline mutations in the TP53* gene. (lifetime risk = 90%) |
|
|
Cowden syndrome: Characterized by high rate of breast cancer and mucocutaneous findings, thyroid disorders and endometrial carcinomas. Associated germline mutations in the PTEN* gene. (lifetime risk = 50%) |
|
|
BRCA1/2 mutations
Nat Rev Clin Oncol. 2010 Dec;7(12):702-7. J Cell Sci. 2001 Oct;114(Pt 20):3591-8. Nat Rev Cancer. 2011 Dec 23;12(1):68-78. |
5-10% breast cancer cases are considered directly related to inheritance of mutations in BRCA1 or BRCA2. Women carrying mutations in BRCA1/2 genes have a 50-80% lifetime risk of breast cancer. |
|
BRCA1 gene is located on chromosome 17q21 and is classified as a tumour suppressor gene. It functions as a pleiotropic DNA damage repair protein. Its mutation is associated with basal-like phenotype of breast cancer, high grade III subtype, high mitotic count, and triple negative (ER/PR/HER2) carcinomas |
|
|
BRCA2 gene is located on chromosome 13q12 and also classified as a tumour suppressor gene; though shares no homology with the BRCA1 gene. However, it can bind with BRCA1 to participate in DNA damage response pathway. BRCA2 protein functions as a mediator of the core mechanism of homologous recombination. Its mutations are linked to breast carcinomas that are ER and PR positive. Though rarely associated with basal-like phenotype but still linked to a higher grade (II or III) when compared to age-matched sporadic cases |
|
|
Cells lacking BRCA1/2 are much more sensitive to ionizing radiation, suggesting a role for BRCA1/2 in DNA damage response (DDR), specifically in repairing double-strand breaks (DSB), which is the major lesion inflicted by ionizing radiation. |
Pathogenesis (hormone sensitive)
- N Engl J Med. 2006 Jan 19;354(3):270-82.
- Oncologist. 2003;8(4):307-25.
Gene expression in breast carcinomas
Two different types of estrogen receptors exist, alpha (α) and beta (β) (ERα and ERβ respectively). Various tissues express these receptors with breast, ovaries and the endometrium expressing ERα, while the kidneys, brain, lungs and several other organs expressing ERβ. The role of ERβ in carcinogenesis remains controversial whereas, a clear contribution of ERα protein has been established.
Both ER subtypes carry a DNA binding domain and exist in the nucleus and the cytosol. When estrogen enters the cell, it binds the ER and the complex migrates into the nucleus and leads to the production of transcription proteins that induces changes in the cell. Therefore, due to estrogen’s proliferative properties, its cellular stimulation can have negative consequences in patients expressing large quantities of these receptors intracellularly.
Role of estrogen in breast cancer progression and development
Two major hypotheses attempt to explain the tumorigenic effects of estrogen: (i) genotoxic effects of estrogen metabolites via generation of radicals (initiator) and (ii) the hormonal properties of estrogen inducing proliferation of cancers as well as the premalignant cells (promoter).
Role of Human Epidermal Growth Factor Receptor 2 (HER2)
HER2 belongs to the epidermal growth factor receptor (EGFR) family of proto-oncogenes and currently is not known to have a ligand. However, the protein has been shown to form clusters within the cell membranes in malignant breast tumours. Its mechanism of carcinogenesis remains largely unknown, but overexpression is associated with rapid tumour growth, shortened survival, increased risk of recurrence after surgery, and poor response to conventional chemotherapeutic agents.
Clinical features
- Med Clin North Am. 2008 Sep;92(5):1115-41, x.
- Mayo Clin Proc. 2001 Jun;76(6):641-7; quiz 647-8.
Very few clinical signs and symptoms exist for breast cancer raising the importance of screening tests in diagnosis.
|
Signs and symptoms |
Characteristics |
|
|
|
Benign |
Malignant |
|
Breast mass – a dominant mass is a distinct mass that is asymmetric with the other breast. Benign findings are often associated with cysts, fibroadenomas, or fibrocystic changes. Malignant disease often has abnormal cell proliferation and calcifications, manifesting as a fixed, firm mass with irregular borders. Any suspicious dominant mass should undergo diagnostic tests (see below). |
|
|
|
Nipple discharge – usually benign, but discharge with blood, from a single duct, or associated with breast mass raises probability of cancer. |
|
|
|
Mastalgia (breast pain) – rarely the presenting symptom of breast cancer
Cyclic mastalgia: Hormonal changes during the menstrual cycle trigger an increase in breast size and volume. |
|
|
|
SOB/dyspnea |
Metastatic disease to the lungs |
|
|
Bone pain and symptoms of hypercalcemia |
Bone metastasis |
|
|
Abdominal distention and jaundice |
Liver and peritoneal metastasis |
|
|
Altered cognitive function and local neurological signs |
Brain metastasis |
|
Diagnosis
- J Natl Compr Canc Netw. 2009 Nov;7(10):1060-96.
Multistep approach:
1) Patients are identified by screening (see below) or symptoms (see above).
2) Imaging is done by either ultrasound or mammography.
- Mammogram has a false negative rate of 11%. It is accurate in detecting calcifications as well as small non-palpable lesion in postmenopausal women with non-dense breast tissue.
- Ultrasound is better at detecting fluid-filled lesions (cysts) and small tumours in dense breast tissue.
3) Biopsy or fine needle aspiration is done if a lump is detected by imaging or if clinically it appears suspicious.
4) Pathological diagnosis distinguishes benign and malignant breast disease.
- Staging is done using the TNM system (see Introduction to neoplasia chapter), but molecular markers (see above) correlate better with prognosis.
Screening
- CMAJ. 2011 Nov 22;183(17):1991-2001.
Evidence
- Screening with mammography every 2-3 years for women of average risk (i.e. no family history, no BRCA mutations) from age 40-74 is associated with reduced mortality.
- Women at increased risk of breast cancer should undergo regular screening by imaging and breast examination at a younger age.
- Screening with clinical breast examination or breast self-examination is not associated with reduction in mortality.
Current Canadian guidelines recommend the following for women of average risk
- No routine screening for women aged 40-49 due to high number of false positives on mammogram and unnecessary biopsies compared to mortality benefit.
- Routine mammography every 2-3 years for women aged 50-74 due to mortality benefit outweighing false positive and unnecessary biopsy risk.
- Routine breast self-examination and clinical breast examinations are not recommended.
- Discussion of this data with patients allows more autonomy for a personalized screening schedule.
Treatment
- Lancet. 2005 May 14-20;365(9472):1727-41.
Surgery
CMAJ. 1998 Feb 10;158 Suppl 3:S15-21.
- Breast-conserving surgery (BSC): also known as lumpectomy or wide local excision, BSC involves resection of the tumour along with a margin of tissue while conserving the cosmetic appearance of the breast. Most breast surgeries are of this type because (i) most tumours are locally invasive and (ii) large primary tumours can be reduced in size by neoadjuvant chemotherapy prior to conservative surgery.
- Mastectomy: surgical removal of entire breast, including the fascia over the pectoralis muscles. Surgeons may preserve some skin and the nipple/areola for reconstruction. The indication for mastectomy is multicentric invasive carcinoma, inflammatory carcinoma, or extensive intraductal carcinomas.
- Axillary lymph node dissection: removal of the lymph nodes draining the breast tissue for lymph node micrometastasis. This is done at the same time as BSC or mastectomy. However, recent evidence suggests that axillary lymph node biopsy is unnecessary regardless of whether the sentinel lymph node biopsy is negative or positive because there is no mortality benefit.
Ann Surg Oncol. 2010 Oct;17 Suppl 3:343-51. - Adjuvant therapy: cytotoxic chemotherapy, endocrine therapy, or radiation therapy may be used postsurgery to prevent relapse.
Radiation therapy
- Either whole or partial breast irradiation may be used (see Carcinogenesis chapter for mechanism of radiation therapy). Adjuvant radiation therapy is applied post-BCS or post-mastectomy to prevent recurrence. Since most recurrence of early-stage breast cancer occurs locally, partial irradiation at the tumour site has similar mortality benefits as whole breast irradiation. However, new evidence suggests an increased risk of local and axillary recurrence with partial irradiation.
Breast J. 2010 May-Jun;16(3):245-51. - Radiation of metastatic disease (e.g. bone or brain metastases) is also used.
Endocrine therapy
Best Pract Res Clin Endocrinol Metab. 2004 Mar;18(1):1-32.
- Breast cancer is a hormone-sensitive cancer. Most breast cancer cells are ER-positive, and thus will respond to reduction of circulating estrogens. HR-negative breast cancers will not respond to endocrine therapy.
- Mainly used as (i) adjuvant therapy for early-stage hormone-sensitive breast cancer or as (ii) first line therapy for metastatic hormone-sensitive breast cancer.
- Cancer Care Ontario recommends 5 years of adjuvant endocrine therapy for early-stage breast cancer in postmenopausal women.
- Antiestrogens (e.g. tamoxifen): Competitively binds ER and inhibits estrogen binding.
- Aromatase inhibitors: Aromatase, also known as estrogen synthase, is an enzyme responsible for estrogen synthesis. There are two types: steroidal (type I) and non-steroidal (type II). The steroidal type (e.g. exemestane) is an androgen analogue that binds permanently with the aromatase enzyme, leading to long-term and specific inhibition of the enzyme. The non-steroidal type (e.g. anastrozole and letrozole) originates from an anti-epileptic drug that reversibly binds and inhibits the cytochrome P450 unit in aromatase. Because the non-steroidal type has a good molecular fit with the substrate-binding site, it is more potent than the steroidal type. Both types have good efficacy and high specificity for the aromatase enzyme.
- Ovarian ablation: induction of artificial menopause by ovariectomy significantly reduces breast cancer risk. Adrenalectomy eliminates a source of androgens in females, which is the precursor to aromatase-derived estrogens. However, these surgical approaches are irreversible and cause major side effects, so they are less often used.
- Ovarian suppression: LHRH (GnRH) agonist (e.g. goserelin and leuprorelin) can be used to reversibly suppress LH/FSH release and thus estrogen release.
Chemotherapy
- Cytotoxic drugs, such as cyclophosphamide, methotrexate, doxorubicin, and paclitaxel, are used in hormone receptor-negative or HER2-positive breast cancers. They can either be given presurgery as neoadjuvant to shrink the tumour or postsurgery as adjuvant to prevent relapse.