Overview
- N Engl J Med. 2000 Aug 3;343(5):338-44.
- Trends Immunol. 2001 May;22(5):235-6.
- Nat Immunol. 2012 Feb 16;13(3):214-22.
- Medicine. 2009 Oct 37(10): 510-517
- Jpn J. Infec Dis. 2004 57: 236-247.
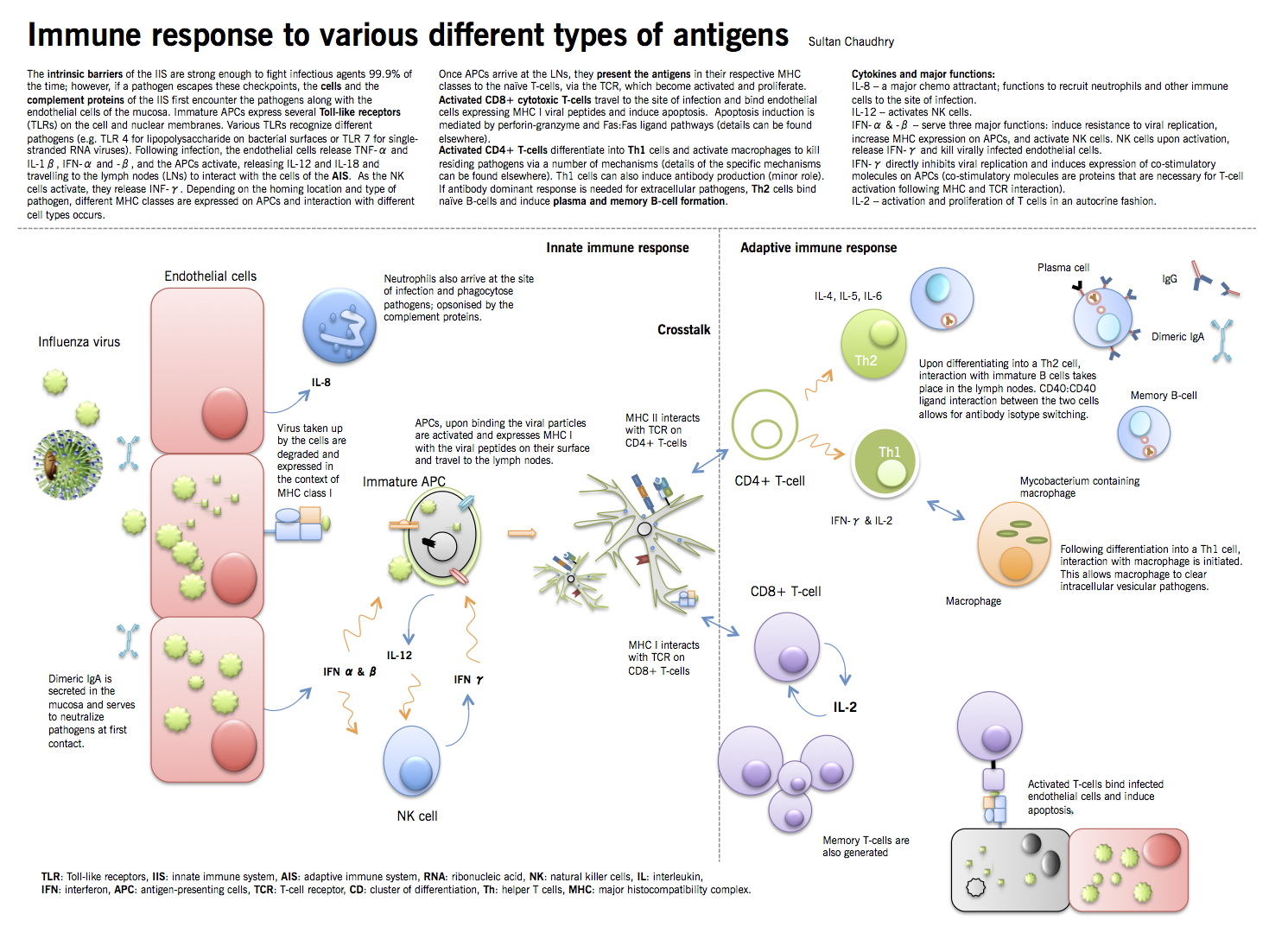
Microorganisms that cause disease in humans and animals enter the body at different sites and produce disease symptoms by a variety of mechanisms. Microbial invasion is initially countered by innate defenses that preexist in all individuals and begin to act within minutes following encounter with the infectious agent. Only when the innate defenses are bypassed, evaded or overwhelmed is an adaptive immune response required. The innate immune system (IIS) is usually sufficient to prevent the body from being routinely overpowered by these organisms. However, once they have gained a hold, they require the concerted efforts of both the IIS and the adaptive immune system (AIS). In the first part of this chapter, different arms and principles of the IIS and the AIS will be briefly discussed. The second part of the chapter will discuss the process of fighting a bacterial and a viral infection, with an emphasis on the cross talk between the two parts of the immune system.
|
Table 1: Innate vs. adaptive immune system |
|
|
Innate immunity |
Adaptive immunity |
|
Immediate response |
Slow/delayed response (3-5 days). Takes time 72-96 hours to generate specific T-cells and generate antibody. |
|
Lack of memory |
Builds memory of prior exposures. Antigen specific memory cells generate a stronger and faster response on re-exposure. |
|
Receptors are encoded in the genome |
Receptors are generated based on prior exposure. T- and B-cells only respond to the specific antigens presented by the APCs (see text for details). |
|
Receptors recognize general patterns of pathogens (pathogen-associated molecular patterns, PAMPs) |
Receptors identify very specific sequences, i.e. epitopes. A large repertoire of receptors on different clones is able to distinguish between different amino acid sequences. |
|
IIS receptors are pattern recognition receptors (PRRs) |
Receptors are B- and T-cell receptors (BCR and TCR, respectively). |
|
Born with most of it (innate) |
Acquired later in life. |
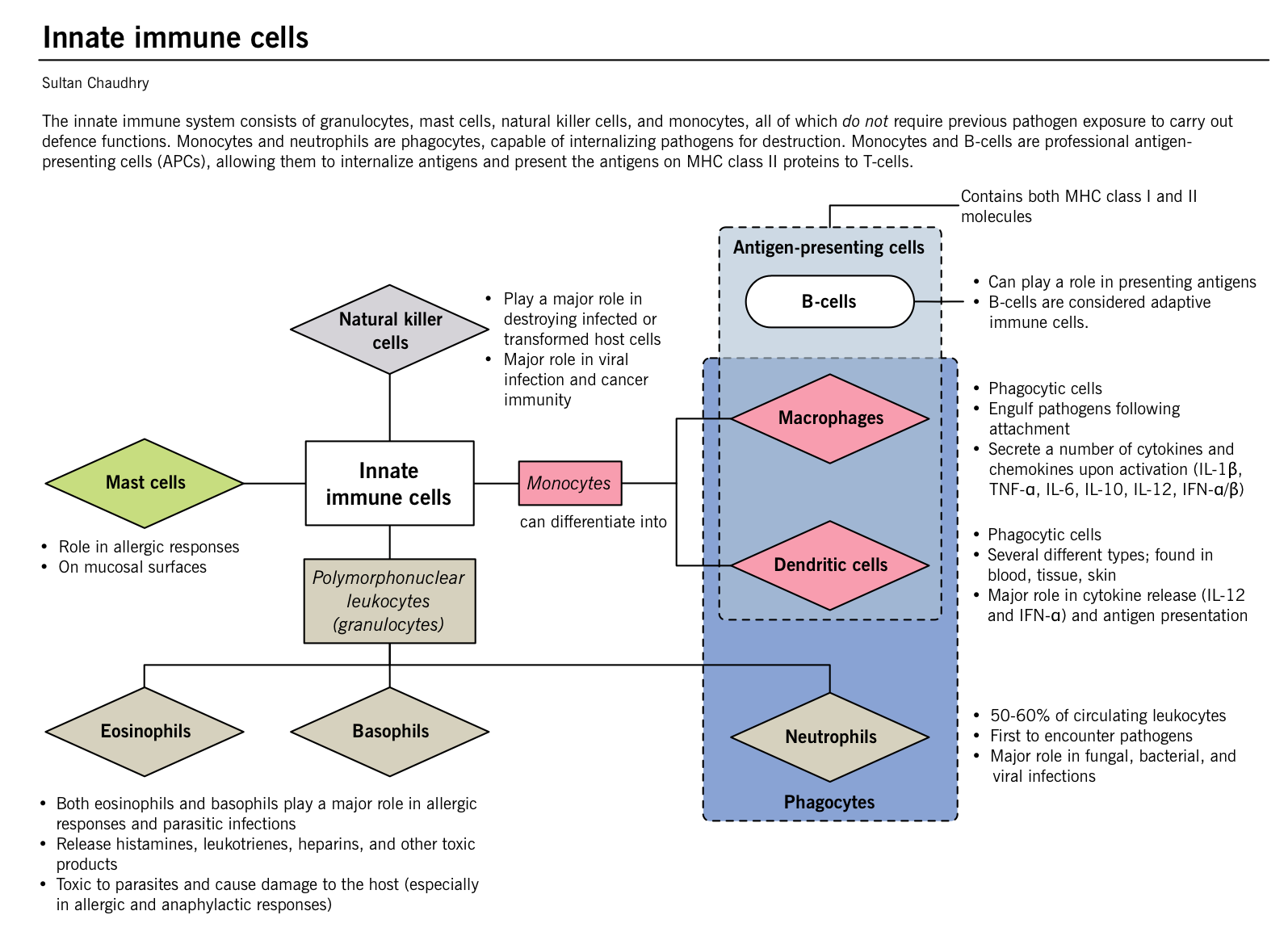
Innate immune system
- Front line of defense for the human body.
- Different components of the IIS include:
- Intrinsic barriers (Table 2)
- Small molecules or peptides: the complement proteins (Figure 1)
- Innate immune cells
Intrinsic barriers
|
Table 2: Intrinsic barriers of the innate immune system |
||
|
Types |
Function |
|
|
Physical/ anatomical barriers |
Tight junctions |
In the epidermal skin layer: water-proof and blocks UV |
|
Subcutaneous glands |
In the dermis: contain fatty acids maintaining a pH of 3-5 |
|
|
Mucous membranes |
Traps microbes |
|
|
Mechanical removal |
Mucus and cilia |
In the respiratory tract: microorganism trapping and attachment prevention by the mucus |
|
Cilia propels the mucus and trapped microbes towards the sites of removal |
||
|
Cough and sneeze reflex |
Respiratory tract: removal of microorganisms from the body |
|
|
Vomiting and diarrhea |
GI tract: removal of toxins and pathogens from the GI tract |
|
|
Flushing of body fluids |
Systemic: fluids such as tears, urine, saliva and sweat also flush microbes from the body |
|
|
Physiological barriers |
Temperature |
Normal body temperature slows down the growth of some pathogens |
|
Elevated body temperature (fever) inhibits growth of pathogens that survive normal body temperature; also causes the release of heat shock proteins, which enhances the immune response. |
||
|
pH |
Low stomach, skin and vaginal pH inhibit microbial growth |
|
|
Chemical barriers |
Lysozymes |
Bactericidal enzyme secreted by the cells, found at the mucosal surfaces |
|
Lactoperoxidase |
Mucosal secretion that stimulates cells to produce toxic radicals |
|
|
Cryptidins and |
Base of crypt cells in the small intestine: damage cell membranes |
|
|
b-defensins |
Produced within the skin, respiratory tract: damage cell membranes |
|
|
Surfactant proteins A and D |
Present in lungs: function as opsonins, enhancing the phagocytic activity of cells. |
|
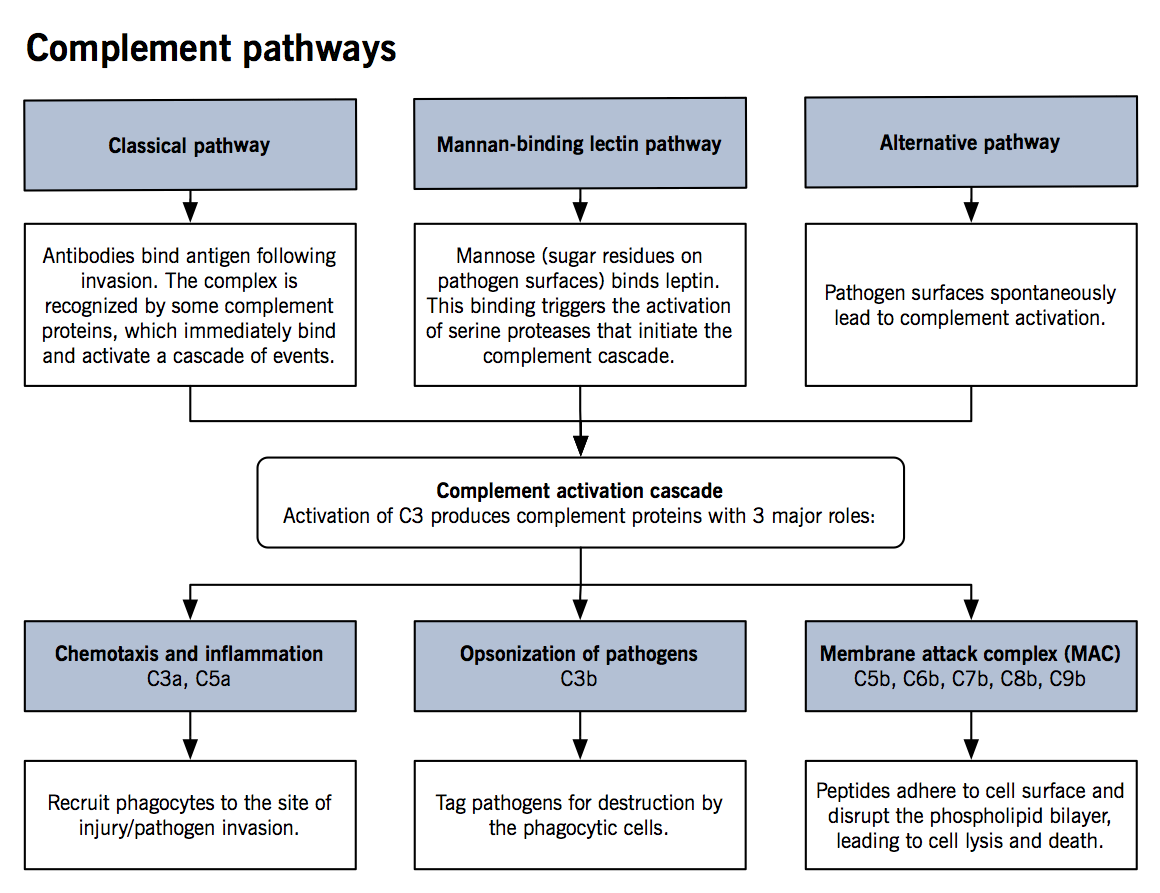
The complement system
- The complement system is comprised of small proteins synthesized by the liver that enhance the strength and activity of an innate as well as the adaptive immune response.
- See Figure for details of activation mechanisms
- Upon activation of C3, a cascade of events takes place where each precursor is cleaved into two parts (C3 à C3a and C3b, C4 à C4a and C4b and so on) until C9a and C9b are generated (naming is due to their activation in the sequence).
- Studies have shown that phagocytic properties of neutrophils and macrophages are significantly enhanced in the presence of complement proteins. Furthermore, chemotaxis by cytokines is upregulated and bacterial killing is stronger with different proteins of the complement system.
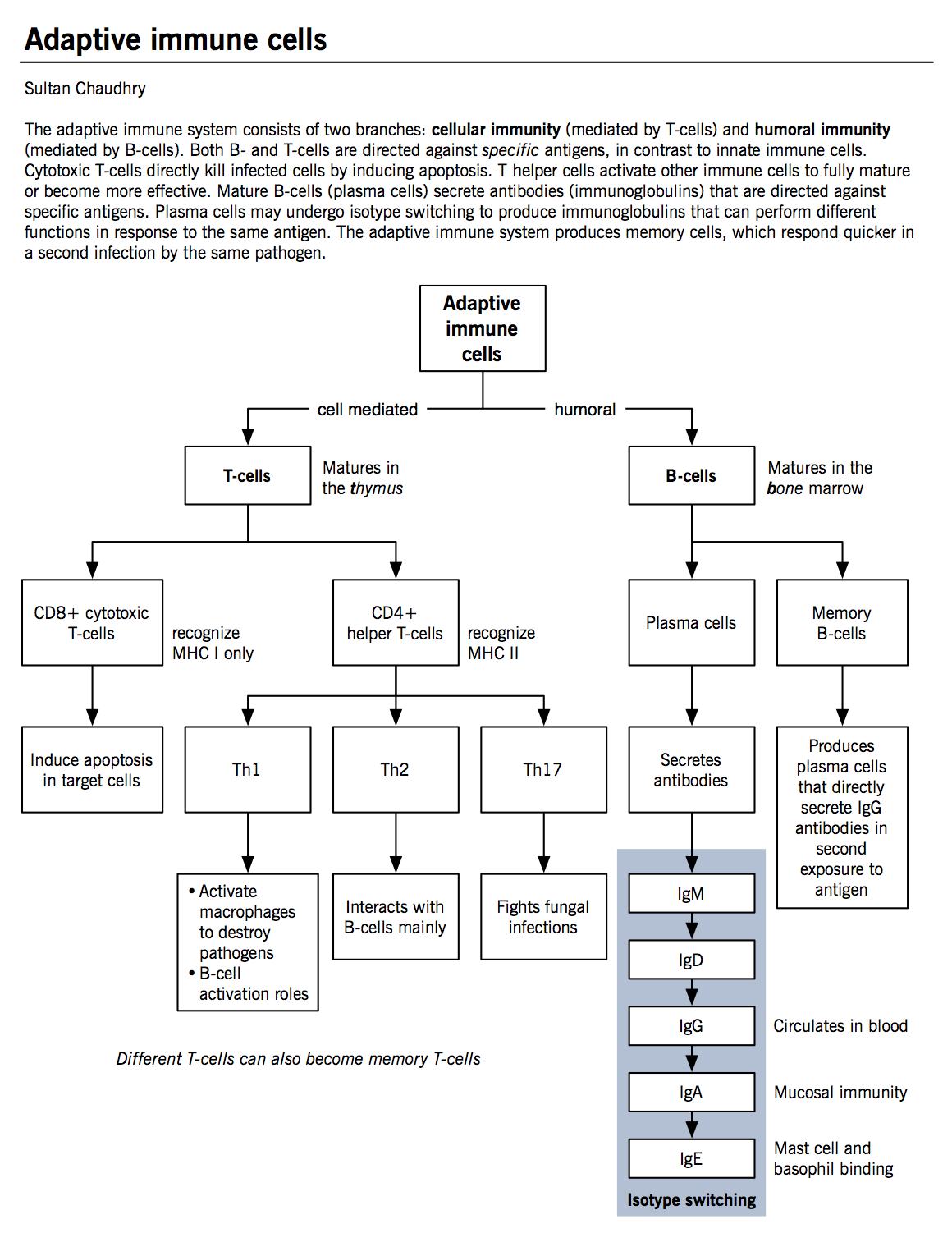
Adaptive immune system
Acquired immunity (or adaptive immunity), is mediated by various T- and B-cells (see Figure for classification), in conjunction with the APCs.
The adaptive immune cells
T-lymphocytes (T-cell)
- Produced in the bone marrow but mature in the thymus.
- Have a unique antigen receptor, generated during development in the thymus. This T-cell receptor (TCR) recognizes a specific peptide sequences bound to the major histocompatibility complex (MHC) on APCs and the infected cells. This TCR and MHC interaction allows T-cells to activate and proliferate.
- MHC is a cell surface molecule that mediates interaction of immune cells with other immune cells or body cells. In humans, MHC is also called human leukocyte antigen (HLA).
- Each person inherits up to 6 class I molecules and 12 class II molecules. Each different type of HLA molecule binds a different antigen sequence for presentation.
- MHC class I molecules are found on all nucleated cells of the body. They present cytosolic protein sequences, and cytotoxic T cells are main responding cell.
- In contrast, MHC class II molecules are only found on lymphoid tissue cells. They present proteins found in lysosomes and endosomes or extracellular pathogens, and T helper cells are the responding cells.
- T-lymphocytes can be classified by their functional characteristics.
- CD8+ or cytotoxic T lymphocytes kill cells infected with viruses, specifically recognizing sequences presented in the context of MHC class I molecule. Once APCs activate T-cells, they travel to the site of infection to recognize the same sequence as presented by the APC on an infected host cell. Upon binding, a number of mechanisms allow for apoptosis induction within the target cell.
- CD4+ T helper cells secrete soluble factors to help other T-cells, B-cells and macrophages. They bind and recognize pathogens in the context of MHC class II molecules.
- Th1 cells play a major role in macrophage activation. They represent the dominant helper cell type during a strong cell-mediated response (e.g. mycobacterium). Th1 cells secrete IL-2 (induces T-cell proliferation) and IFN-γ (stimulates macrophage and NK cell activation).
- Th2 cells play a dominant role in B-cell activation and antibody production. Their interaction is necessary for isotype switching in B-cells (a process whereby a B-cell builds different antibody isotypes from the same backbone; IgG, IgA, or IgE, from IgM). Th2 cells secrete IL-4 and IL-5 (induce IgE synthesis and eosinophil activation during parasitic infections) and IL-10 (inhibits Th1 cell proliferation).
- Th17 cells play a major role in fungal infections (see fungal infections chapter). Th17 cells secrete IL-17 (activates neutrophils to kill fungal cells/hyphae at the site of infection).
- Each person inherits up to 6 class I molecules and 12 class II molecules. Each different type of HLA molecule binds a different antigen sequence for presentation.
|
Table 3: Classification, function, and major secretions of CD4+ T helper cells. |
|||
|
Major function |
Type of immune response |
Major cytokines |
|
|
Th1 cells |
Macrophage and NK cell activation |
Cell mediated immune response |
IL-2 and IFN-γ (directly cause macrophage and NK cell activation |
|
Th2 cells |
B-cell activation and antibody production |
Humoral immune response |
IL-4, IL-5, and IL-6 (induce IgE synthesis and eosinophil activation during parasitic infections). IL-10 (inhibit Th1 cell and induce Th2 cell proliferation) |
|
Th17 cells |
Neutrophil activation |
Cellular response to fungal infections |
IL-22 (induces secretion of defensins that lyse the fungal cell wall) and IL-17 (recruits neutrophils to the site of infection). |
|
IL: interleukin, Ig: immunoglobulin, Th: T helper cell, NK: natural killer cells, IFN: interferon |
|||
B-lymphocytes (B-cells)
- Produced and mature in the bone marrow.
- Play the lead role in humoral immunity and mainly functions to produce various types of antibody. Several different types of B-cells exist but only the major ones are discussed here: memory B-cells and plasma cells.
- Memory cells are formed from the activated B-cells specific for a certain antigen encountered during an active immune response. These cells remain in the circulation for long periods of time and respond immediately upon the exposure of the same antigen.
- Plasma cells produce and secrete copious amounts of various antibodies. Each plasma cell is specific for a specific antibody (one plasma cell cannot secrete all antibodies).
- B-cell activation can be T-cell dependent (see Figure) or it can also be T-cell independent. Since MHC can present peptide sequences only, carbohydrate sequences directly bind B-cells and cross-link antibody receptors (antibodies can act as B-cell receptors also) leading to B-cell activation.
- Antibodies are large protein molecules secreted by B-cells. They are comprised of two chains: heavy chains and light chains (κ and λ). Different antibodies serve a number of functions. (see Table 4)
|
Table 4: Antibody significance, characteristics and functions |
|||||
|
IgM (pentamers and hexamers) |
IgD (monomer) |
IgG (monomer) |
IgA (dimer) |
IgE (monomer) |
|
|
Features |
|
|
|
|
|
|
Neutralization |
+++ |
+++ |
|||
|
Opsonization |
+++ |
+ |
|||
|
ADCC |
+++ |
||||
|
Mast cell sensitization |
+++ |
||||
|
Complement activation |
+++ |
+++ |
+ |
||
|
Ig: immunoglobulin, ADCC: antibody-dependent cell cytotoxicity ADCC is a process whereby an immune cell lyses or induces apoptosis in an antibody-bound target cell. Classic ADCC is mediated by the natural killer cells (via IgG; in any infectious state) and eosinophils (via IgE during a parasitic infection). |
|||||
Activation of the adaptive immune system
Major determinant of which immune arm will be activated by a certain pathogen depends on where the pathogen resides: cytosol, intracellular endosomes, or in the extracellular environment.
|
Table 5: Type and residence of pathogens and the adaptive arm generating defense. |
|||
|
|
Cell-mediated immunity |
Humoral immunity |
|
|
Typical pathogens |
Vaccinia virus Influenza virus Rabies virus Listeria |
Mycobacterium tuberculosis Leishmania donovani Pneumocystis jirovecii |
Clostridium tetani Staphylococcus aureus Streptococcus pneumoniae Poliovirus Pneumocystis jirovecii |
|
Location |
Cytosol |
Macrophage vesicles |
Extracellular fluid |
|
Effector T-cells |
Cytotoxic CD8+ T-cells |
Th1 CD4+ T-cells |
Th1/ Th2 CD4+ T-cells |
|
Antigen recognition |
Peptide: MHC I on infected cells |
Peptide: MHC II on infected macrophages |
Peptide: MHC II on B-cells |
|
Effector action |
|
|
|