Cardiac conducting system
Clin Anat. 2009 Jan;22(1):99-113.
Can J Anaesth. 1993 Nov;40(11):1053-64.
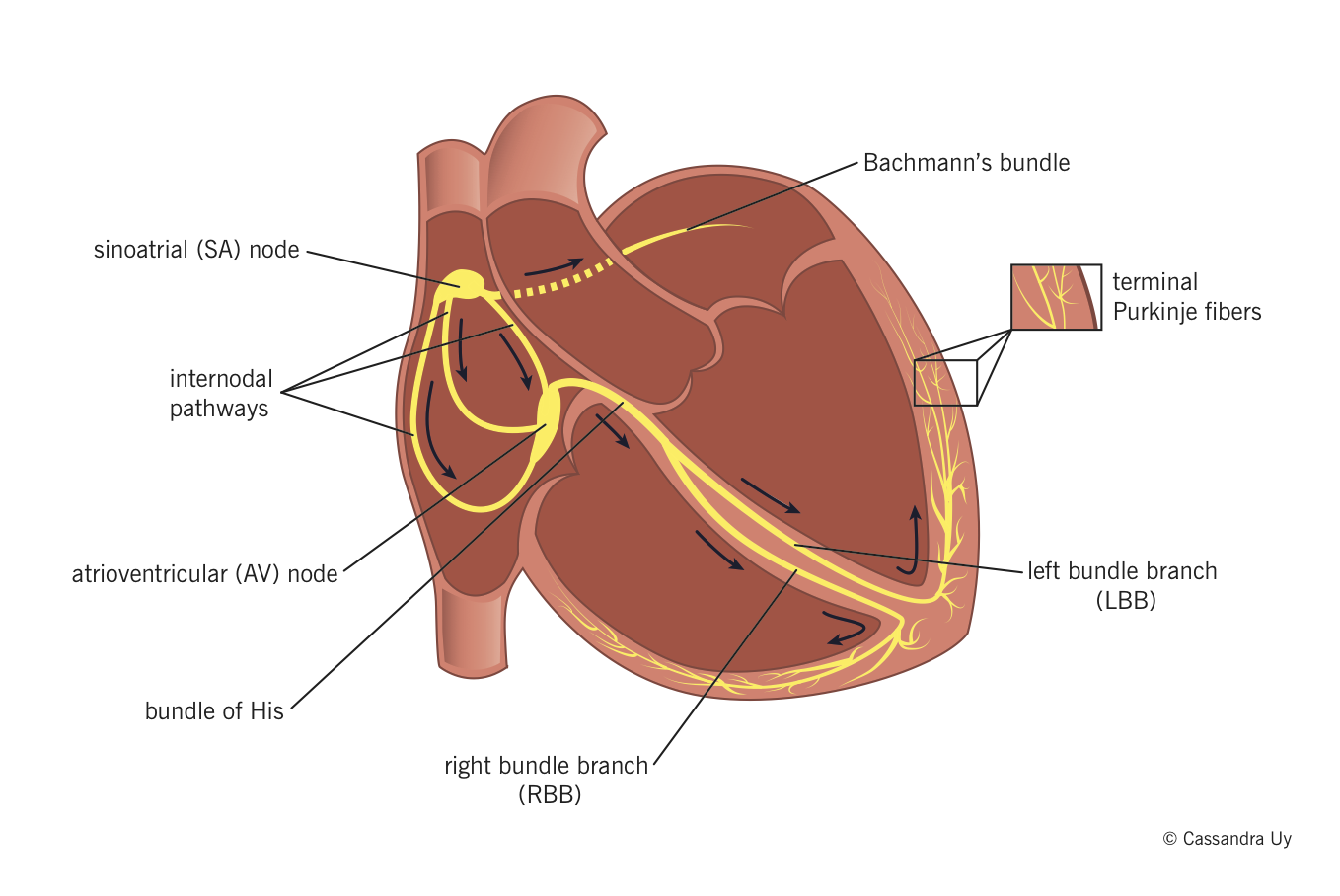
- Sinoatrial (SA) node normally generates the action potential, i.e. the electrical impulse that initiates contraction.
- The SA node excites the right atrium (RA), travels through Bachmann’s bundle to excite left atrium (LA).
- The impulse travels through internodal pathways in RA to the atrioventricular (AV) node.
- From the AV node, the impulse then travels through the bundle of His and down the bundle branches, fibers specialized for rapid transmission of electrical impulses, on either side of the interventricular septum.
- Right bundle branch (RBB) depolarizes the right ventricle (RV).
- Left bundle branch (LBB) depolarizes the left ventricle (LV) and interventricular septum.
- Both bundle branches terminate in Purkinje fibers, millions of small fibers projecting throughout the myocardium.
An organized rhythmic contraction of the heart requires adequate propagation of electrical impulses along the conduction pathway. Of note, the impulses in the His-Purkinje system travel in such a way that papillary muscle contraction precedes that of the ventricles, thereby preventing regurgitation of blood flow through the AV valves.
Electrophysiology
Physiol Rev. 2005 Oct;85(4):1205-53.
Ion channels
Heart Rhythm. 2010 Jan;7(1):117-26.
- Two main forces drive ions across cell membranes:
- Chemical potential: an ion will move down its concentration gradient.
- Electrical potential: an ion will move away from ions/molecules of like charge.
- The transmembrane potential (TMP) is the electrical potential difference (voltage) between the inside and the outside of a cell. When there is a net movement of +ve ions into a cell, the TMP becomes more +ve, and when there is a net movement of +ve ions out of a cell, TMP becomes more –ve.
- Ion channels help maintain ionic concentration gradients and charge differentials between the inside and outside of the cardiomyocytes.
Properties of cardiac ion channels
- Selectivity: they are only permeable to a single type of ion based on their physical configuration.
- Voltage-sensitive gating: a specific TMP range is required for a particular channel to be in open configuration; at all TMPs outside this range, the channel will be closed and impermeable to ions. Therefore, specific channels open and close as the TMP changes during cell depolarization and repolarization, allowing the passage of different ions at different times.
- Time-dependence: some ion channels (importantly, fast Na+ channels) are configured to close a fraction of a second after opening; they cannot be opened again until the TMP is back to resting levels, thereby preventing further excessive influx.
Note: The different types of cardiac ion channels are discussed below, throughout the description of the phases of action potentials in different cardiac cells.
Action potentials and impulse conduction
Physiol Rev. 2005 Oct;85(4):1205-53.
Action potential: electrical stimulation created by a sequence of ion fluxes through specialized channels in the membrane (sarcolemma) of cardiomyocytes that leads to cardiac contraction.
Action potential in cardiomyocytes
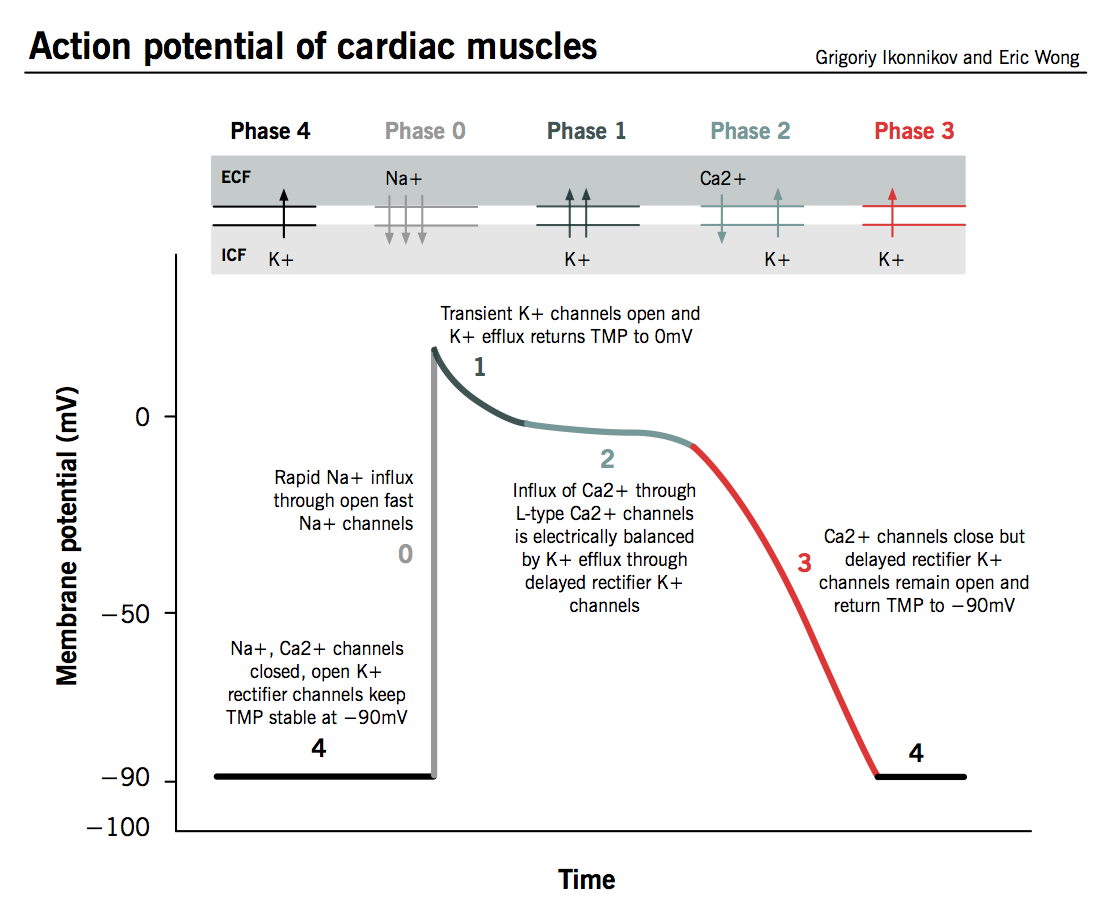
The action potential in typical cardiomyocytes is composed of 5 phases (0-4), beginning and ending with phase 4.
Phase 4: The resting phase
- The resting potential in a cardiomyocyte is −90 mV due to a constant outward leak of K+ through inward rectifier channels.
- Na+ and Ca2+ channels are closed at resting TMP.
Phase 0: Depolarization
- An action potential triggered in a neighbouring cardiomyocyte or pacemaker cell causes the TMP to rise above −90 mV.
- Fast Na+ channels start to open one by one and Na+ leaks into the cell, further raising the TMP.
- TMP approaches −70mV, the threshold potential in cardiomyocytes, i.e. the point at which enough fast Na+ channels have opened to generate a self-sustaining inward Na+ current.
- The large Na+ current rapidly depolarizes the TMP to 0 mV and slightly above 0 mV for a transient period of time called the overshoot; fast Na+ channels close (recall that fast Na+ channels are time-dependent).
- L-type (“long-opening”) Ca2+ channels open when the TMP is greater than −40 mV and cause a small but steady influx of Ca2+ down its concentration gradient.
Phase 1: Early repolarization
- TMP is now slightly positive.
- Some K+ channels open briefly and an outward flow of K+ returns the TMP to approximately 0 mV.
Phase 2: The plateau phase
- L-type Ca2+ channels are still open and there is a small, constant inward current of Ca2+. This becomes significant in the excitation-contraction coupling process described below.
- K+ leaks out down its concentration gradient through delayed rectifier K+ channels.
- These two countercurrents are electrically balanced, and the TMP is maintained at a plateau just below 0 mV throughout phase 2.
Phase 3: Repolarization
- Ca2+ channels are gradually inactivated.
- Persistent outflow of K+, now exceeding Ca2+ inflow, brings TMP back towards resting potential of −90 mV to prepare the cell for a new cycle of depolarization.
- Normal transmembrane ionic concentration gradients are restored by returning Na+ and Ca2+ ions to the extracellular environment, and K+ ions to the cell interior. The pumps involved include the sarcolemmal Na+-Ca2+ exchanger, Ca2+-ATPase and Na+-K+-ATPase.
Action potential in cardiac pacemaker cells
Pharmacol Ther. 2005 Jul;107(1):59-79.
Drugs. 2007;67 Suppl 2:15-24. (The funny current)
- Automaticity: unlike other cardiomyocytes, pacemaker cells do not require external stimulation to initiate their action potential; they are capable of self-initiated depolarization in a rhythmic fashion. This property is known as automaticity, whereby the cells undergo spontaneous depolarization and an action potential is triggered when threshold voltage is reached.
- Unstable membrane potential: Pacemaker cells have an unstable membrane potential and their action potential is not usually divided into defined phases.
- No rapid depolarization phase: Pacemaker cells have fewer inward rectifier K+ channels than do other cardiomyocytes, so their TMP is never lower than −60 mV. As fast Na+ channels need a TMP of −90 mV to reconfigure into an active state, they are permanently inactivated in pacemaker cells so there is no rapid depolarization phase.
Table 1. Cardiac cell types displaying pacemaker behavior.
|
Pacemaker |
Location |
Inherent rate (beats per minute, BPM) |
|
Sinoatrial (SA) node
|
Right atrium (RA) at junction with superior vena cava (SVC) |
60-100 BPM |
|
Atrioventricular (AV) node |
RA at posteroinferior area of interatrial septum |
40-60 BPM |
|
Purkinje fibers and ventricular cardiomyocytes |
Throughout the ventricles |
20-40 BPM |
The sequence of events for pacemaker action potential:
- Spontaneous flow of ions mainly through slow Na+ channels slowly depolarizes TMP above −60 mV. This is called the funny current (also known as pacemaker current); it is active at TMPs of less than −55 mV.
- At TMP −55 mV, T-type Ca2+ channels open and continue slow depolarization.
- TMP −40 mV is the threshold potential for pacemaker cells. L-type Ca2+ channels open and depolarize cell to 0 mV, then overshoot to +40 mV.
- Delayed rectifier K+ channels counteract the L-type Ca2+ channels for a brief plateau phase and then return the TMP back to −60 mV as Ca2+ channels close.
Implications of pacemaker activity on global cardiac depolarization
- Synchronous contraction: all cardiomyocytes (including pacemaker cells) are electrically coupled through gap junctions. An action potential in one cell will cause all neighbouring cells to depolarize, allowing the heart chambers to act as a unit.
- Dominance: the cell with the highest inherent rate of pacemaker activity will therefore also set the heart rate, as all other pacemaker cells will be depolarized and rendered inactive by this stimulus.
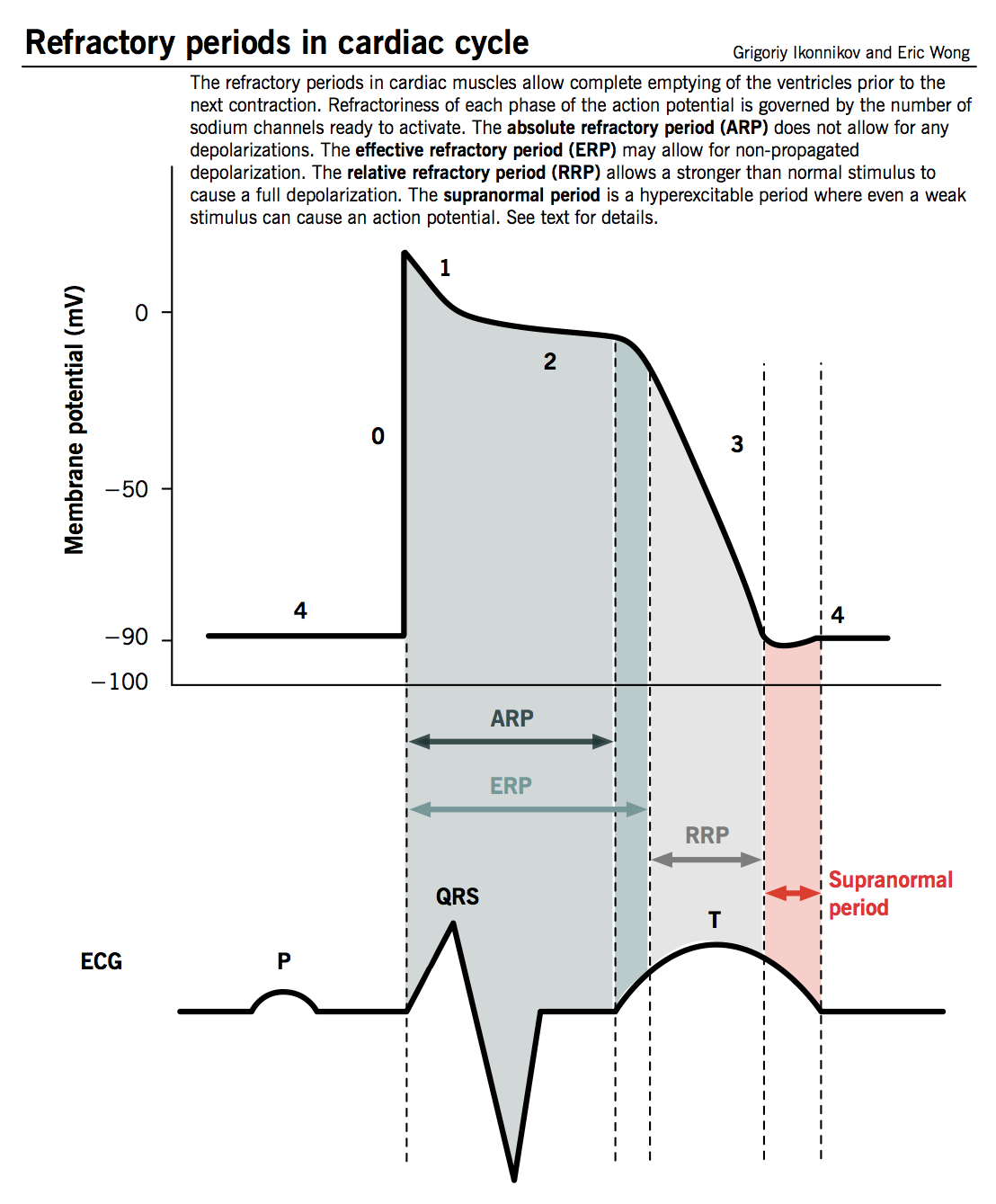
Refractory period
- Defined as the time from phase 0 until the next possible depolarization of a myocyte, i.e. once enough fast Na+ channels have recovered (as TMP decreases below −50 mV).
- Cardiomyocytes have a longer refractory period than other muscle cells given the long plateau from slow Ca2+ channels (phase 2). This is a physiological mechanism allowing sufficient time for the ventricles to empty and refill prior to the next contraction.
- Different degrees of refractoriness are encountered during an action potential, reflecting the number of fast Na+ channels that have recovered from their inactive state and are capable of reopening.
- Absolute refractory period (ARP): the cell is completely unexcitable to a new stimulus.
- Effective refractory period (ERP): ARP + short segment of phase 3 during which a stimulus may cause the cell to depolarize minimally but will not result in a propagated action potential (i.e. neighbouring cells will not depolarize).
- Relative refractory period (RRP): a greater than normal stimulus will depolarize the cell and cause an action potential.
- Supranormal period: a hyperexcitable period during which a weaker than normal stimulus will depolarize the cells and cause an action potential. Cells in this phase are particularly susceptible to arrhythmias when exposed to an inappropriately timed stimulus, which is why one must synchronize the electrical stimulus during cardioversion to prevent inducing ventricular fibrillation.
Sequence of depolarization
- The SA node normally initiates electrical activation.
- The impulse propagates through atrial tissue to the AV node. There is no direct electrical connection between the atrial and ventricular chambers other than through the AV node, as fibrous tissue surrounds the tricuspid and mitral valves. AV node allows a very short delay in conduction (approximately 0.1 second) because it is composed of slower conducting fibers. This pause has two important purposes:
- Allows the atria time to contract and fully empty prior to ventricular stimulation.
- Allows the AV node to act as a gatekeeper, limiting the transmission of ventricular stimulation during abnormally rapid atrial rhythms.
- After crossing the AV node, the impulse spreads into the rapidly conducting bundle of His and through the bundle branches to the Purkinje fibers. The electrical impulse is distributed throughout the bulk of the ventricular myocyte for precisely timed stimulation and contraction of the ventricles.
Excitation-contraction coupling
Nature. 2002 Jan 10;415(6868):198-205.
Excitation-contraction coupling represents the process by which an electrical action potential leads to contraction of cardiac muscle cells. This is achieved by converting a chemical signal into mechanical energy via the action of contractile proteins.
Calcium is the crucial mediator that couples electrical excitation to physical contraction by cycling in and out of the myocyte’s cytosol during each action potential.
Contractile proteins
Main contractile elements:
- Myosin: thick filaments with globular heads evenly spaced along their length; contains myosin ATPase.
- Actin: smaller molecule (thin filaments) consisting of two strands arranged as an alpha-helix, woven between myosin filaments.
Regulatory elements:
- Tropomyosin: double helix that lies in the groove between actin filaments. It prevents contraction in the resting state by inhibiting the interaction between myosin heads and actin.
- Troponin: complex with three subunits that sits at regular intervals along the actin strands.
- Troponin T (TnT) – ties troponin complex to actin and tropomyosin molecules.
- Troponin I (TnI) – inhibits activity of ATPase in actin-myosin interaction.
- Troponin C (TnC) – binds calcium ions that regulate contractile process.
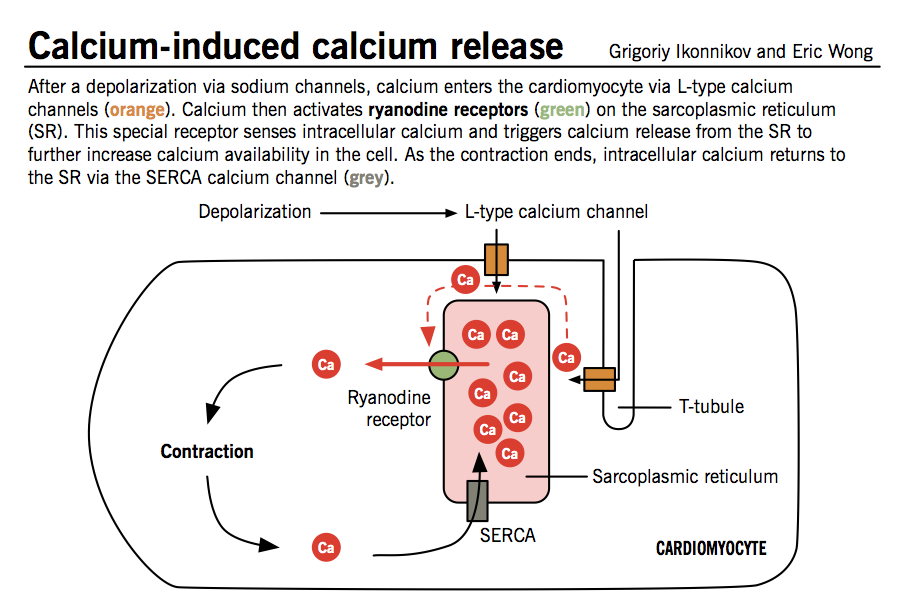
Calcium-induced calcium release (CICR)
The initial influx of Ca2+ into myocytes through L-type Ca2+ channels during phase 2 of the action potential is insufficient to trigger contraction of myofibrils. This signal is amplified by the CICR mechanism, which triggers much greater release of Ca2+ from the sarcoplasmic reticulum.
- The cell membrane of cardiomyocytes, called sarcolemma, contains invaginations (T-tubules) that bring L-type Ca2+ channels into close contact with ryanodine receptors, specialized Ca2+ release receptors in the sarcoplasmic reticulum (SR).
- When Ca2+ enters the cells through L-type channels, ryanodine receptors change conformation and induce a larger release of Ca2+ from abundant SR stores.
- Large levels of intracellular Ca2+ act on tropomyosin complexes to induce myocyte contraction.
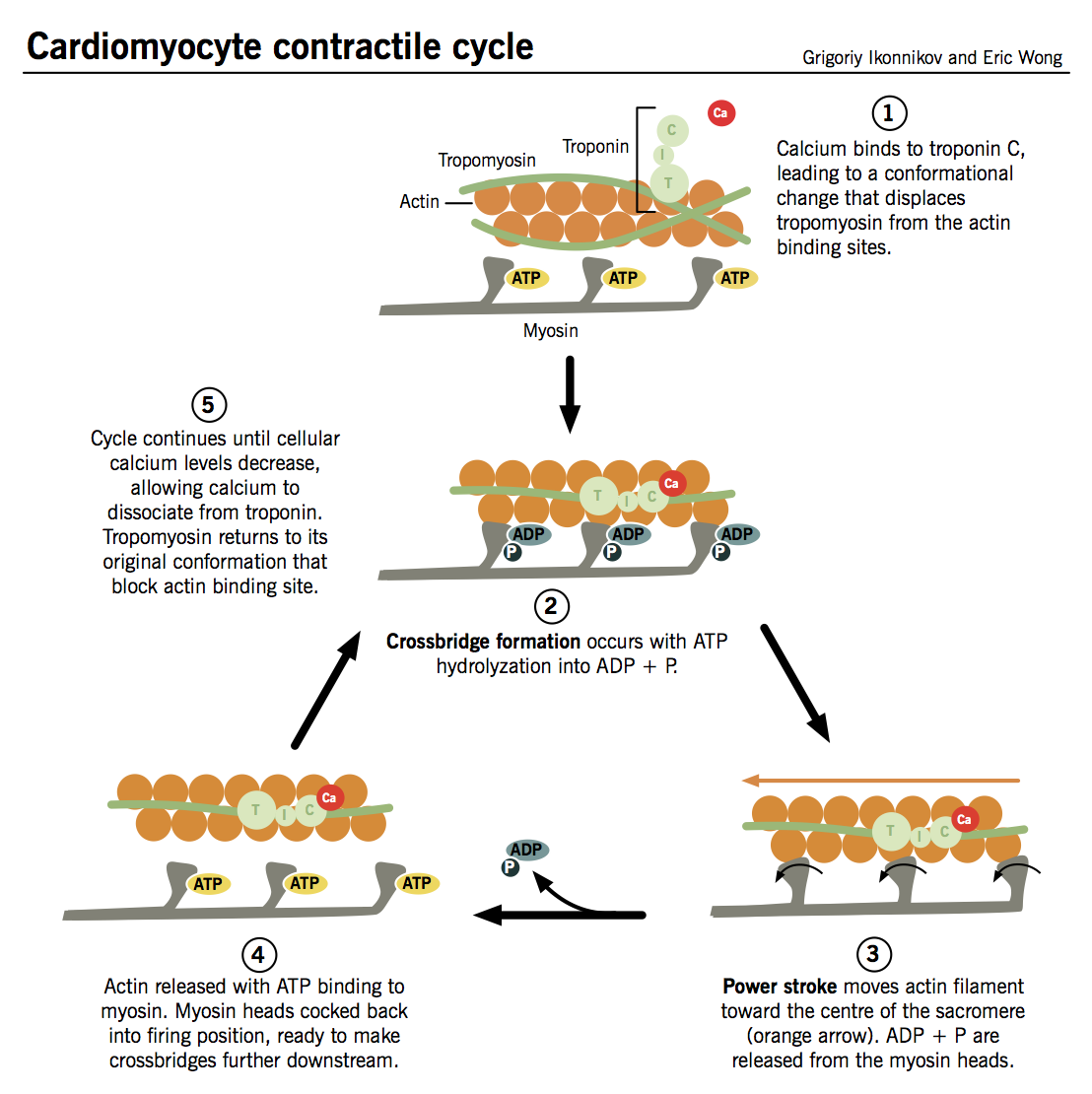
Contractile cycle
- Ca2+ binds to TnC → TnI is inhibited → conformational change in tropomyosin that exposes active site between actin and myosin.
- Myosin heads interact with active sites on actin filaments and “flex,” like oars on a boat, to “row” myosin along actin in an ATP-dependent reaction:
- Hydrolysis of ATP by ATPase on myosin (no longer inhibited by TnI) induces crossbridge formation between myosin head and active site on actin. The strength of cardiac contraction is proportional to the number of crossbridges formed.
- Interaction between myosin head and actin trigger “firing” of myosin head, causing it to pull itself along the actin filament in a process known as the power stroke.
- ADP is released from the myosin head, which then binds a new ATP, releasing the actin filament.
- The cycle can then repeat itself, allowing myosin to travel further along the actin molecules and progressively shorten the muscle fibers, as long as (i) the cytosolic Ca2+ concentration remains sufficiently high to inhibit the action of TnI and (ii) there is enough ATP to drive crossbridge formation.
Myocyte relaxation
As with myocyte contraction, this process is synchronized with the electrical activity of the cell.
- L-type Ca2+ channels inactivate toward the end of phase 2 → Ca2+ influx arrests → CICR trigger is abolished.
- At the same time, Ca2+ is sequestered back into the SR by sarcoplasmic reticulum Ca2+ ATPase (SERCA) and pumped out of the cell to a lesser extent by specialized Ca2+ pumps.
- Ca2+ ions dissociate from TnC as their intracellular concentration falls, and tropomyosin inhibition of actin-myosin interaction is restored.
Neural modulation of contractility
Adv Physiol Educ. 2011 Mar;35(1):28-32.
- Heart is innervated by both parasympathetic and sympathetic afferent and efferent neurons.
- Sympathetic: postganglionic sympathetic fibers from paravertebral sympathetic ganglia associated with T1-T5 innervate the atria, ventricles, and conduction system.
- Parasympathetic: parasympathetic innervation is limited to vagal efferent fibers which innervate the SA node and the AV node; parasympathetic innervation to the ventricles is minimal.
- Both sympathetic and parasympathetic tone is exerted on the heart at rest, but parasympathetic tone predominates.
- Sympathetic neurons release norepinephrine, a catecholamine, which activates β1 receptors on cardiac myocytes, leading to the following effects (note: epinephrine, also a catecholamine, can be made by the adrenal glands and released into the circulation, and has the same effect on β1 receptors):
- Chronotropic: increased heart rate
- Dromotropic: faster conduction through AV node
- Inotropic: increased contractility
- Lusitropic: faster relaxation after contraction
- Parasympathetic neurons release acetylcholine, a cholinergic hormone, which activates muscarinic M2-receptors on cardiac myocytes, leading to just one main effect:
- Negative chronotropic: decreased heart rate