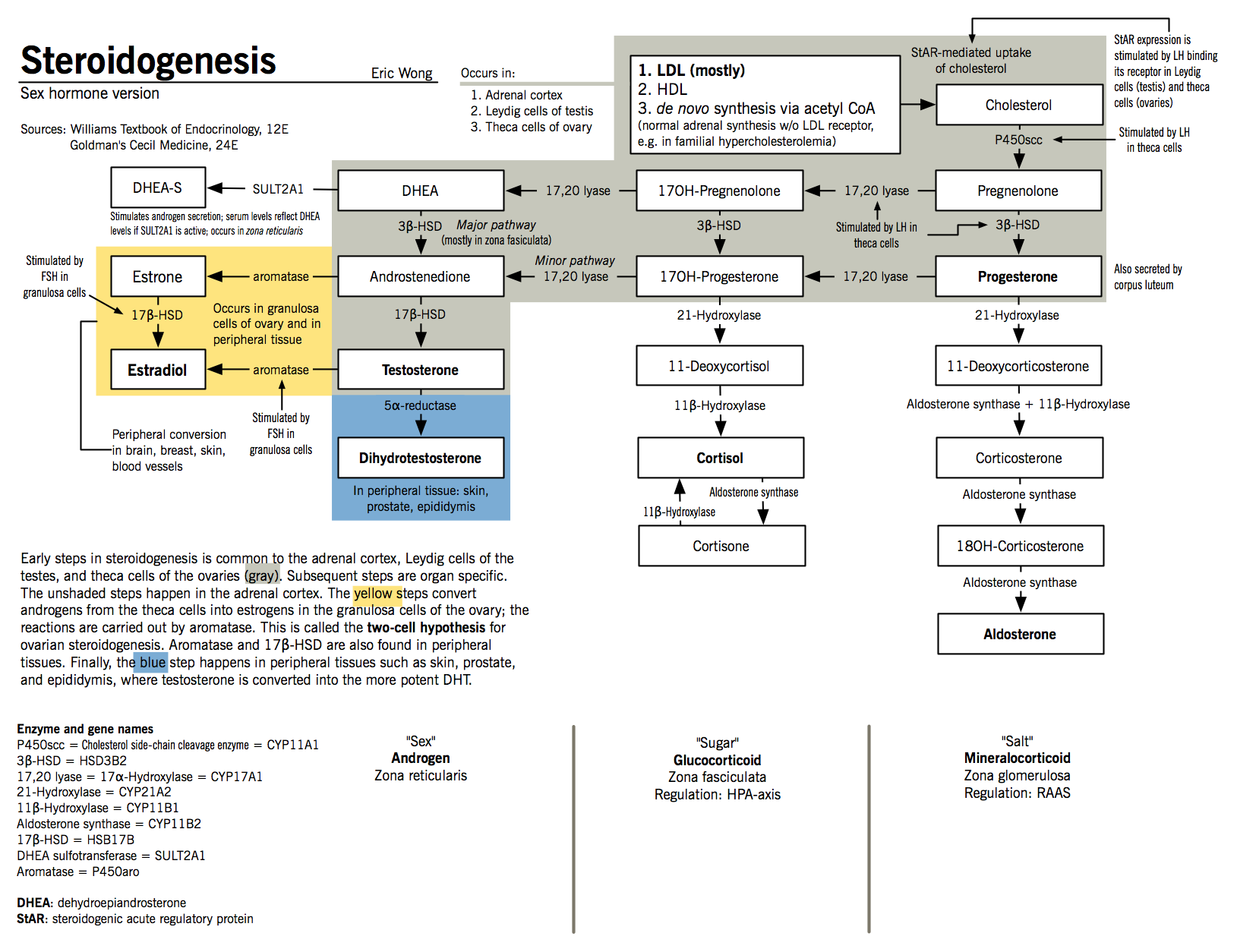
Sex hormone synthesis
- Annu Rev Physiol. 2001;63:193-213.
- Endocr Rev. 2011 Feb;32(1):81-151.
- Endocr Rev. 2005 May;26(3):322-30.
- Fertil Steril. 2002 Apr;77 Suppl 4:S3-5.
The hypothalamus-pituitary-gonadal (HPG) axis
- Sex hormone synthesis is controlled by the pulsatile release of hypothalamic gonadotropin-releasing hormone (GnRH)
- At the pituitary gland, GnRH stimulates the release of luteinizing hormone (LH) and follicle-stimulating hormone (FSH) into the general circulation
- LH then binds to its target cells (Leydig cells in males and theca cells in females) and increases the expression of steroidogenic acute regulatory protein (StAR)
- StAR promotes the transfer of cholesterol to the inner mitochondrial membrane and initiates steroidogenesis.
- This is the rate-limiting step of steroidogenesis in all tissues.
- At the inner mitochondrial membrane, cholesterol is converted to pregnenolone by the action of P450scc.
Androgen synthesis in males
- Androgens are steroid hormones that control the expression and maintenance of male sexual characteristics
- Adrenal androgens DHEA and androstenedione are produced in the zona reticulata and zona fasciculata of the adrenal cortex.
- Testosterone is produced Leydig cells, which are found adjacent to the seminiferous tubules of the testes
- In Leydig cells, LH initiates the production of pregnenolone
- Pregnenolone is then converted to DHEA in a two-step process mediated by 17,20-lyase (17α-hydroxylase)
- Because Leydig cells express high levels of 3β-HSD and 17β-HSD, DHEA is rapidly converted to testosterone via the intermediates androstenediol and androstenedione
- Testosterone is converted to dihydrotestosterone (DHT) by the action of 5α-reductase in target tissues; although it is about one-tenth as abundant as testosterone, it accounts for most of testosterone’s biological action
Androgen synthesis in females
- Although androgens are typically considered the male hormones, they also play important physiologic roles in females
- Active androgens are largely created from circulating precursors in their target tissues, where they both act and are metabolized
- Androgen precursors present in females include:
- DHEA sulfate (DHEAS), produced by the zona reticularis of the adrenal glands;
- DHEA, produced by the zona reticularis, ovarian theca cells, and peripherally from circulating DHEAS;
- Androstenedione, produced by the zona fasiculata of the adrenal glands, the ovarian stroma, and peripherally from circulating DHEA
- About 50% of testosterone in females is produced from circulating precursor molecules, with the other half synthesized in the zona reticularis and the ovarian stroma
- DHT is also produced in females, but circulates in low concentrations in serum and is largely produced in peripheral target tissues
- Testosterone, but not DHT, is converted to estradiol by the action of aromatase (P450aro) in certain peripheral tissues, and may be an important source of estrogens in postmenopausal women
Estrogen synthesis in females
- Estrogens are a class of steroid hormones which control the development and maintenance of female sexual characteristics
- Glandular estrogen synthesis: occurs in the granulosa and theca cells of the ovaries, as well as the corpus luteum
- The granulosa cells are stimulated by LH to produce pregnenolone
- Pregnenolone diffuses out of these cells to adjacent theca cells
- Theca cells express 17,20-lyase and 3β-HSD, which mediate the conversion of pregnenolone to androstenedione via DHEA
- Most androstenedione returns to the granulosa cells and is converted to estrone by aromatase, which is then converted to estradiol by 17β-HSD
- The expression of aromatase and 17β-HSD is controlled by FSH stimulation
- The granulosa cells are stimulated by LH to produce pregnenolone
- Extraglandular synthesis: Aromatase is expressed in non-gonadal sites and facilitates peripheral aromatization of androgens to estrone.
- Fat cells: increases serum estrogens by converting androgen to estrone.
- Bone: converts testosterone to local estrogen to help mature the epiphyses.
Estrogen synthesis in males
- Although estrogens are responsible for female sexual characteristics, they are also synthesized in males.
- Certain peripheral target tissues express aromatase, which facilitates the conversion of circulating testosterone to estradiol and androstenedione to estrone.
- These estrogens are thought to act locally and be metabolized in target tissues, which limits their systemic effects.
- Sites of aromatase expression in the male include the reproductive tract, particularly in Leydig cells, Sertoli cells, and mature spermatocytes; the bone, particularly osteoblasts and chondrocytes; and in adipose tissues.
Progestin synthesis
- Progesterone is synthesized from pregnenolone by action of 3β-HSD in the corpus luteum, by the placenta during pregnancy; as well as by the adrenals, as a step in androgen and mineralocorticoid synthesis.
- Its actions are primarily mediated by an intracellular progesterone receptor, whose numbers increase in the presence of estrogen.
- The products of hormone synthesis vary with the menstrual cycle; estradiol is the main product during follicular maturation, whereas progesterone is the main product in the luteal phase following ovulation.
Sex hormone regulation
- Asian J Androl. 2005 Mar;7(1):3-20.
- Eur J Endocrinol. 1999 Feb;140(2):111-29.
- Guyton 12e Chapter 80, 81
The HPG axis and hormonal feedback in males
- The pulsatile release of GnRH from the hypothalamus causes the secretion of the gonadotropins LH and FSH (to a lesser extent) into the circulation.
- LH stimulates the release of testosterone from Leydig cells.
- Testosterone feeds back negatively on pituitary LH and hypothalamic GnRH.
- This effect may be mediated by estradiols generated by peripheral aromatisation of testosterone.
- FSH stimulates the release of inhibin from Sertoli cells.
- Inhibin feeds back only on anterior pituitary release of FSH.
- LH stimulates the release of testosterone from Leydig cells.
The HPG axis and hormonal feedback in females
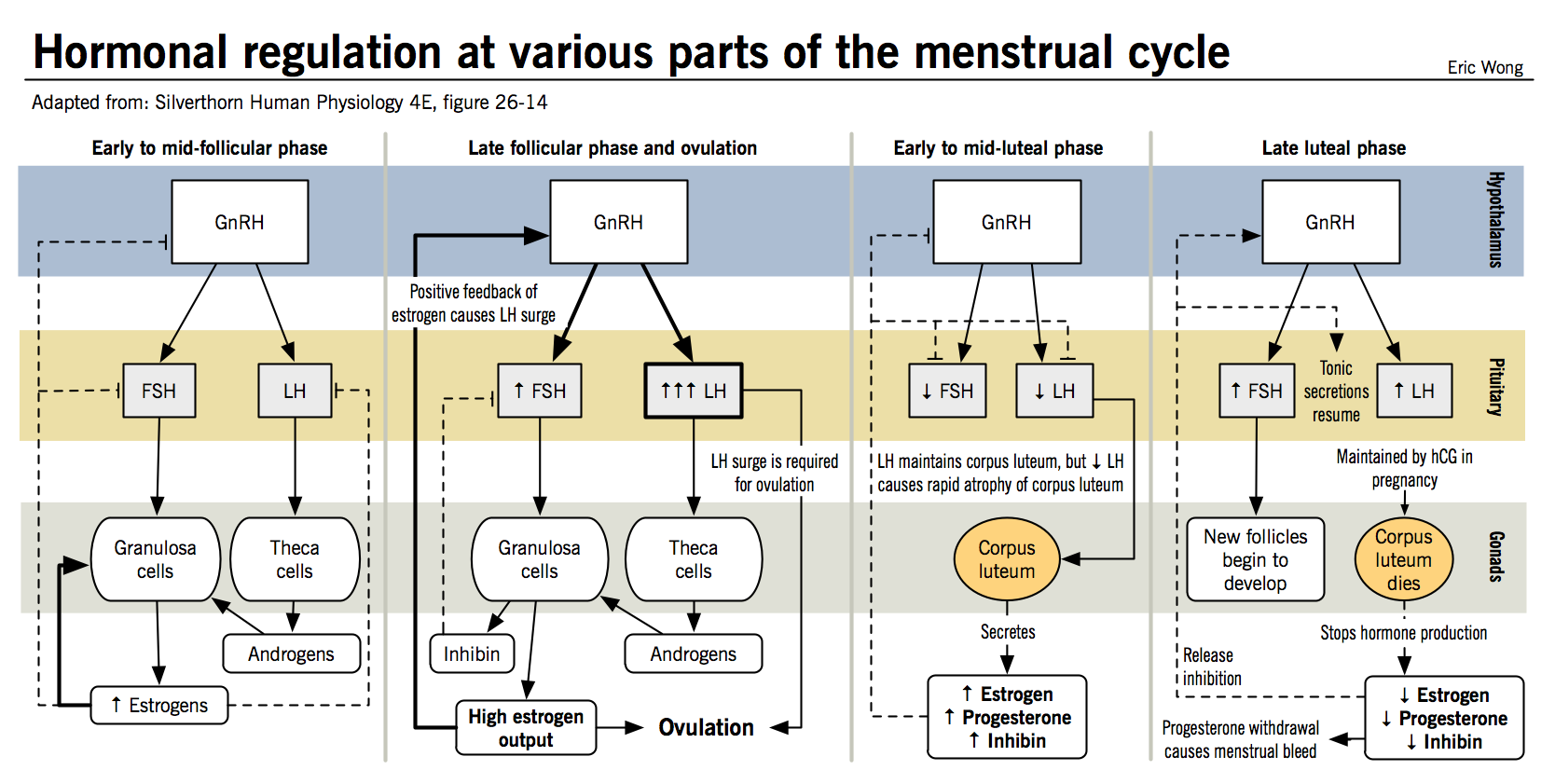
- Hormonal regulation in females is more complicated than in males because the feedback effects of different hormones vary with the stage of the menstrual cycle
- As in males, GnRH released by the hypothalamus results in the release of LH and FSH at the anterior pituitary
- Feedback from theca cells occurs via the release of progestins, whereas feedback from granulosa cells occurs via the release of inhibin and estradiol; progesterone and estradiol are also released by the corpus luteum in the post-ovulatory phase of the menstrual cycle
- Inhibin always acts to inhibit the release of FSH from the anterior pituitary
- Estradiol and progesterone may have stimulator or inhibitory activities, depending on the stage of the menstrual cycle:
- Postovulatory phase: Large amounts of progesterone and estrogen are released by the corpus luteum and inhibit LH and FSH release
- Late luteal phase: FSH and LH levels begin to rise in response to regression of the corpus luteum and a decrease in estrogen and progesterone, leading to the recruitment of a new follicle.
- Follicular growth phase: the growing follicle then begins releasing estrogen in increasing amounts, which has a mildly inhibitory effect on LH and FSH release
- Preovulatory surge: LH and FSH increase suddenly in preparation for ovulation; it is unclear exactly how this occurs, but it is thought that the switch from inhibition to stimulation is either the result of further increases in estradiol production or the consequence of the new follicular progesterone secretion.
Sex hormone effects
Estrogens
- Endocrinology. 2011 Dec;152(12):4481-8.
- Endocr Rev. 2005 May;26(3):322-30.
- N Engl J Med. 2002 Jan 31;346(5):340-52.
Estrogens have a variety of effects on both the sexual organs and diverse target tissues. Although they play different roles in normal male and female physiology, they do in some cases have analogous activities in both sexes.
|
Effect |
Men |
Women |
|
Female sexual characteristics: estrogens promote the development of breast tissue and the growth and differentiation of the sexual organs. |
Men with liver disease who have an excess of estrogen because of the inability of their liver to metabolize it develop gynecomastia, palmar erythema, and spider angiomas. |
Estrogen expression is responsible for female primary and secondary sexual characteristics. Estradiol promotes epithelial cell proliferation in the uterine endometrium and mammary glands of the breasts. In the absence of progesterone, endometrial thickening will proceed unopposed, potentially leading to endometrial hyperplasia and cancer. High exposure to estadiol has also been shown to increase risk for certain breast cancers. |
|
Energy homeostasis and metabolism: estrogen deficiency can result in development of the metabolic syndrome. |
In men, aromatase deficiency has been associated with the development of metabolic derangement characterized by increased circulating LDL, decreased glucose tolerance, hyperinsulinemia, and hepatic steatosis. |
In women, estrogen deficiency has been associated with an increase in adipose cell volume and the growth of peripheral fat pads. |
|
Prevention of bone loss: estrogens act on osteoblasts and osteoclasts to decrease resorption of bone through: (1) increasing expression of factors on osteoblasts which promote osteoclast apoptosis; (2) increasing osteoprotegrin expression and decreasing RANK-RANK ligand signalling |
Men with estrogen insensitivity or aromatase deficiency are at increased risk of loss in bone density and osteoporosis. |
Menopause is thought to cause an increase risk of osteoporosis. |
|
Vasoprotection: estrogens may decrease the risk of atherogenesis in males and females. |
Case studies have demonstrated premature atherogenesis in aromatase-deficient men. |
Animal studies have demonstrated a role for estrogen in preventing the formation of new atherosclerotic plaques, but this role has not yet been demonstrated in humans. |
Androgens
- Arch Intern Med. 2006 Jul 10;166(13):1380-8.
- J Clin Endocrinol Metab. 2000 Aug;85(8):2670-7.
- Nature Clinical Practice Endocrinology & Metabolism (2006) 2, 432-433
Testosterone, along with dihydrotestosterone, is responsible for the development of male primary and secondary sexual characteristics. In women, hyperandrogenism can result in aberrant expression of male sexual characteristics; however, a certain level of androgen activity is also needed for normal female physiology.
|
Effect |
Men |
Women |
|
Male sexual characteristics: testosterone promotes the development of the male sexual organs as well as secondary sexual characteristics |
Testosterone and DHT are important for sexual development as well as secondary sexual characteristics such as thick skin and male-pattern hair growth. |
Hyperandrogenism in women causes hirsutism, which is the excessive growth of body hair in areas associated with male-pattern growth such as the chin and upper lip. |
|
Mood, sexual drive and desire: testosterone has a stimulatory effect on libido in both women and men. |
In men, increased testosterone is associated with an increase in sexual drive and the drop in testosterone with age is associated with a decreasing libido. |
In women with adrenal insufficiency and consequent low androgen levels, replacement with DHEA has been found to increase energy and improve libido and sexual thoughts. |
|
Bone formation: testosterone is thought to increase bone thickness and periosteal bone formation. |
The effect of testosterone on bone, via aromatisation to estrogen, is thought to account for increased bone strength in men over women. |
Testosterone increased bone mineral density in women with hypopituitarism but this may have been due to the effects of aromatization to estrogen. |
|
Metabolism: testosterone has an important role in metabolism and muscle deposition. |
Testosterone increases basal metabolic rate and muscle mass. |
In women with hypopituitarism, testosterone supplementation increased fat-free mass and muscle. |
|
Erythropoiesis: testosterone promotes red blood cell formation and protects against anemia. |
Replacing testosterone in males with hypogonadism results in increases in red blood cell mass. |
Low levels of testosterone in older women may increase the risk of anemia. |
|
Estrogen precursor: testosterone and androstenedione can be aromatized to form estrogens. |
In men, peripheral aromatization of testosterone plays an important role in estrogen production in bones and the reproductive tract, where it plays an important role in normal physiology. |
In postmenopausal women, aromatization of circulating androgens is an important source of estrogens and may help to ameliorate some of the consequences of menopause. |
Progesterone
- Endocr Rev. 1997 Aug;18(4):502-19.
- Brain Res Rev. 2006 Jun;51(1):136-43.
Females
Progesterone has several well-defined roles in females.
- Progesterone is secreted by ovarian follicular cells prior to ovulation; it is also secreted in larger amounts by the corpus luteum, which forms from follicular granulosa cells following ovulation.
- The corpus luteum will grow for 10-12 days and then regress if fertilization does not occur; if fertilization does occur, the corpus luteum is maintained for the first 2-3 months of pregnancy.
- Progesterone plays several important actions in the normal female reproductive cycle:
- Prepares the uterus for pregnancy by shifting the endometrium from proliferation to secretion.
- Withdrawal of progesterone in the absence of pregnancy leads to organized shedding (menstruation).
- Helps mediate sexual response in the brain.
- After fertilization, progesterone:
- Organizes the vasculature of the endometrium to prepare for implantation
- Promotes enzymatic digestion of the zona pellucida to allow the oocyte to implant into the uterine wall
- Inhibits contractions of the uterine myometrium (smooth muscle layer) and counteracts the effects of oxytocin on contractility
- Promotes lobuloalveolar growth in the breasts to prepare for lactation, but suppresses premature milk protein synthesis prior to parturition
- Some of the effects of progesterone may be related to its ability to antagonize estrogen by decreasing expression of estrogen receptors, e.g. the ability of progesterone to inhibit estrogen-mediated endometrial proliferation
- Progesterone also has a potent effect as an antagonist of the mineralocorticoid receptor, reducing sodium retention when present, and increasing sodium retention when progesterone is withdrawn.
Males
The role of progesterone in males is less clear, but it is believed to play a role in activating sperm in the female reproductive tract. It has also been implicated as a modulator of male sexual response and behaviour.