Overview
Normal adult hemoglobin (HbA) consists of a tetramer made up of two alpha-globin and two beta-globin subunits. The alpha globin gene is found on chromosome 16 and is duplicated, which means that each somatic cell with its pair of homologous chromosomes contains 4 copies of the alpha chain gene. The gene encoding beta globin only has two copies, one present on each of the pair of chromosome 11.
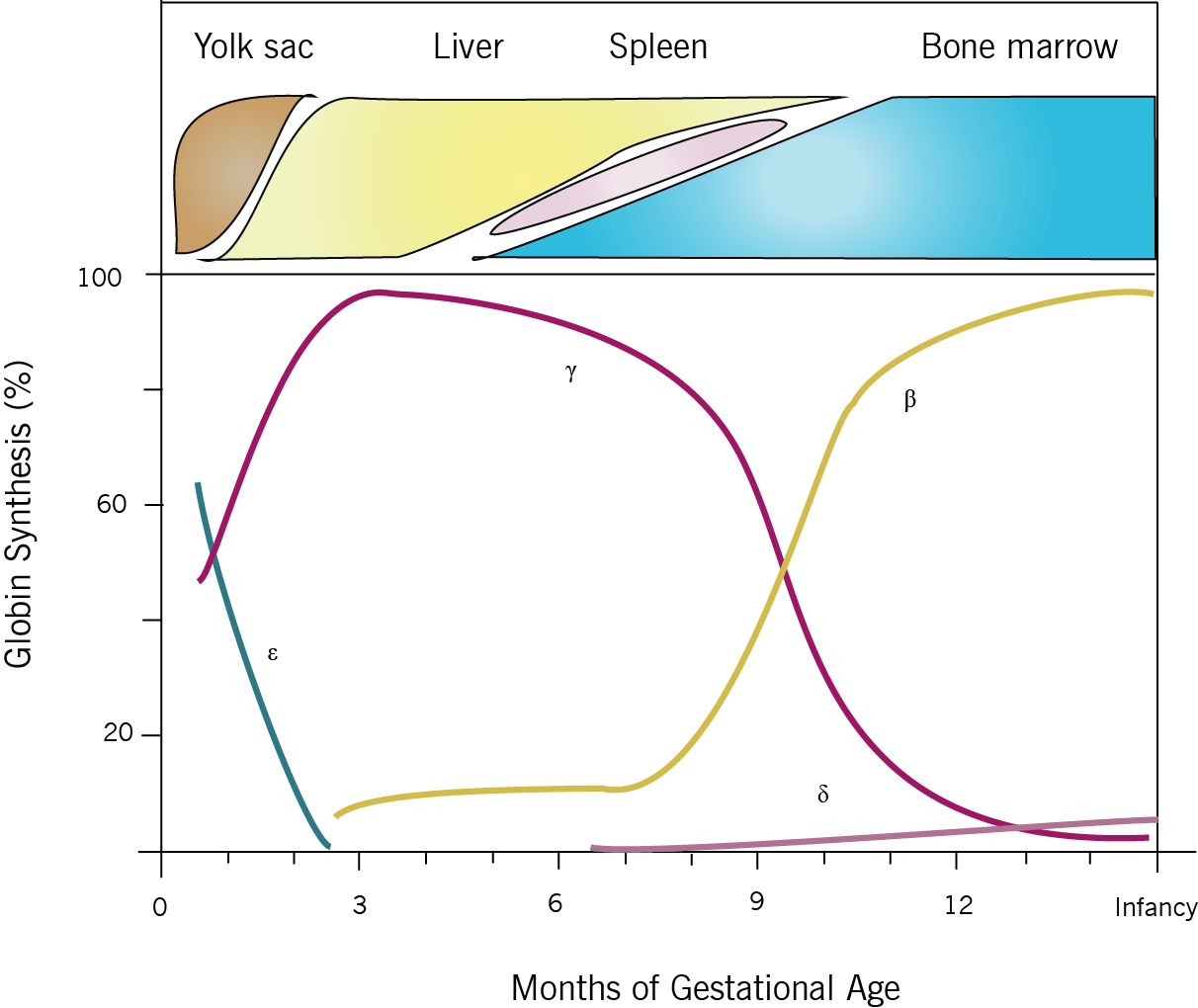
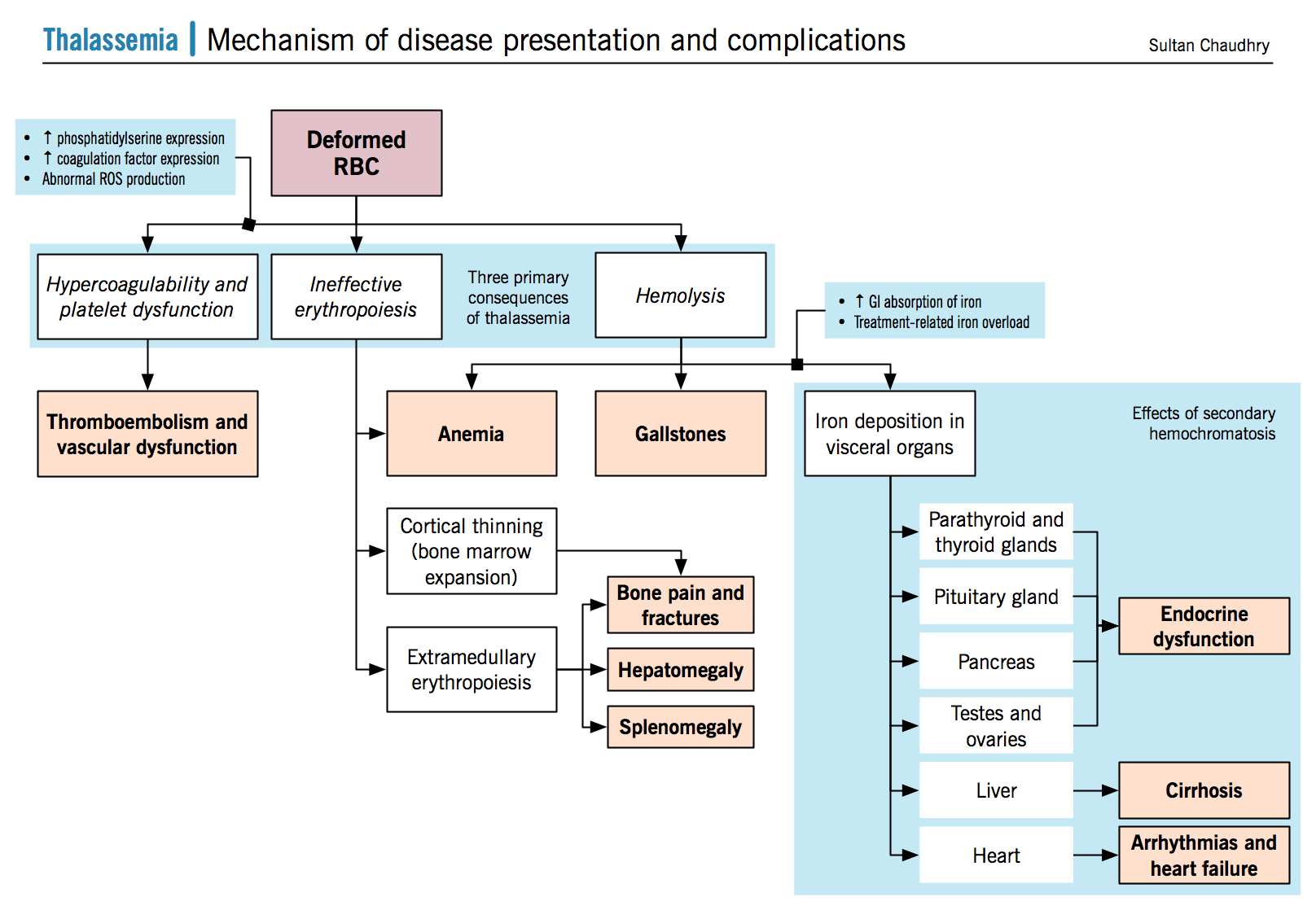
Thalassemia results when mutations affecting the genes involved in Hb biosynthesis lead to decreased Hb production. The clinical phenotype results from both the diminished amount of the particular globin chain as well as from the resultant chain imbalance that occurs because of normal production of the other globin chain. It might be important to note that the clinical phenotype becomes most apparent at 6-9 months of age; due to the fetal to adult hemoglobin switch that occurs at that age (see figure below). The common forms include alpha and beta thalassemias depending on the gene affected.
Beta thalassemia
Genetics/etiology
- Upwards of 100 mutations have been described that decrease beta chain synthesis. Most of these are point mutations, and interfere with processes such as splicing, chain termination, and promoter sites resulting in defective gene transcription or translation.
- Mutations fall into two classes:
- B0 refers to mutations that cause no beta globulin to be produced
- B+ describes mutations that result in a diminished but not absent quantity of beta globulin. The severity of these mutations can vary depending on the amount of normal beta globulin that is produced
- Depending the class of mutation present and the gene dosage (i.e. heterozygous or homozygous) patients can present with differing severity of disease
- Beta thalassemia major: refers to a severe clinical phenotype that occurs when patients are homozygous or compound heterozygous for more severe beta chain mutations (e.g. severe B+/B+ mutations, B+/B0, B0/B0)
- Beta thalassemia intermedia: An in between clinical phenotype with heterogenous genetic mutations that still allow for some Beta chain production (e.g. B+/B0, B+/B+). Some rare cases also exist in which both beta and alpha mutations coexist
- Beta thalassemia minor/ thalassemia trait: a mild clinical phenotype when one normal copy of the beta globulin gene is present (e.g. B+/B, B0/B)
Alpha thalassemia
Genetics/etiology
- Many mutations can affect the alpha globin gene, but the most common are gene deletions
- As mentioned previously, there are 4 copies of the alpha gene in each somatic cell. Thus, phenotypes increase in severity as the number of functional alpha genes decreases
- Silent carrier: refers to patients with one alpha gene deletion, they are clinically asymptomatic. (e.g. a-/aa)
- Alpha thalassemia trait: these patients have two alpha gene deletions, and very mild phenotypes. Gene deletions can both be present on the same chromosome, or divided between the two chromosomes which has relevance for the patients offspring. (e.g. aa/– or a-/a-)
- Hemoglobin H disease: named reflecting the presence of the beta tetramer HbH (B4) found in red cells. Causes moderately severe anemia. Occurs with three alpha chain deletions (e.g. a-/–)
- Hydrops fetalis: the most severe form, caused by 4 alpha gene deletions (e.g. –/–). Becomes manifest later in fetal development, when the fetus transitions from using early embryonic globin alleles (gamma2/zappa2) to later fetal alleles (gamma2/alpha2). Red cells contain gamma-globin tetramers (Hb Bart) which are ineffective at delivering oxygen to tissues, causing anoxia, edema, hepatosplenomegaly. Historically was not compatible with life, but aggressive in-utero and lifelong transfusions may save individuals with this condition
Presentation
|
Signs and symptoms |
Mechanism(s) |
|
Shortness of breath, fatigue, and weakness (anemia) |
Secondary to anemia Deficient synthesis of the beta chain of hemoglobin causes red cells have low HbA levels, thus explaining their phenotype and impaired ability to transport oxygen Imbalance between alpha and beta globin production leads to a precipitation of the relatively overabundant alpha globin chain within the RBCs their precursors |
|
Hepatomegaly |
Ineffective erythropoiesis leads to activation of extramedullary erythropoiesis in areas such as the spleen, liver, lymph nodes, and the thorax. Hepatomegaly can result from a number of mechanisms; extramedullary erthyropoiesis, hepatitis due to chronic transfusion associated infections, and iron overload. |
|
Bone pain and fragility fractures |
Caused by two major mechanisms:
|
|
Cardiac failure and arrhythmias |
Secondary to anemia and iron overload |
|
Splenomegaly |
Secondary to extramedullary hemotopoiesis Can also be due to extravascular hemolysis causing a hypertropic response in the spleen. |
|
Gallstones |
Bilirubin stones
|
|
Malnutrition |
Rapidly growing erythrocyte precursors compete for nutrients and can cause malnutrition |
|
Bronze skin |
Cutaneous iron deposition damages the skin and enhances melanin production by the melanocytes. |
Complications
- N Engl J Med. 2005 Sep 15;353(11):1135-46
|
Complication |
Mechanism |
|
Iron overload (see iron metabolism section) |
A combination of factors, including ineffective erythropoiesis and tissue hypoxia, lead to increased iron absorption via the GI tract; via hepcidin inhibition.
Compounded by life long transfusions Iron deposition occurs in visceral organs (mainly in the endocrine glands, the heart, and the liver) causing failure. |
|
Endocrinopathies |
Due to iron deposition in multiple endocrine glands inducing damage to those glands.
|
|
Hemolysis |
Alpha globin precipitation leads to cell membrane damage
|
|
Cortical destruction and impaired bone growth |
Uncompensated anemia creates a strong stimulus through erythropoietin (EPO) signalling to ramp up erythropoiesis. This causes erythroid hyperplasia within the bone marrow, which expands and destroys adjacent areas of bone such as the cortex, impairing bone growth and creating malformations |
|
Arterial and venous thromboembolism |
RBC membrane dysfunction increases the expression of negatively charged phosphatidylserines, which have been shown to induce platelet activation. Deformed RBCs may directly induce vascular damage RBCs and platelets carry higher levels of ROS (fenton reaction, described above) Further hypothesis include a chronic hypercoaguable state, due to abnormal levels of coagulation factors in thalassemia patients. |
Treatment
- Curr Gastroenterol Rep. 2007 Mar;9(1):74-82.
- Ann N Y Acad Sci. 2010 Aug;1202:237-43.
- Transfusions:
- Regular blood transfusions to ensure non-anemic states and prevent some of the disease complications (Target Hb 90-100 g/L)
- Leukodepletion techniques are used to ensure less alloimmunization and non-hemolytic transfusion reactions.
- Testing for viruses is done to reduce transfusion transmitted infections
- Regular blood transfusions to ensure non-anemic states and prevent some of the disease complications (Target Hb 90-100 g/L)
- Iron chelation:
- Deferoxamine/deferiprone work by binding serum iron and clearing it via the urine.
- Deferiprone has been shown to improve cardiac functioning (left ventricular ejection fraction; LVEF) in patients with thalassemia major.
- Endocrine therapy:
- Administration of the deficient hormones (sex hormones and thyroid hormones)
- Use of fertility agents to induce spermatogenesis and achievement of pregnancy
- osteoclast inhibitors (bisphosphonates) to prevent osteopenia and osteoporosis.
- Splenectomy and cholecystectomy:
- Splenectomies often assist with reducing transfusion requirements
- Cholecystectomies are often required to the presence of bilirubin stones in the gallbladder.